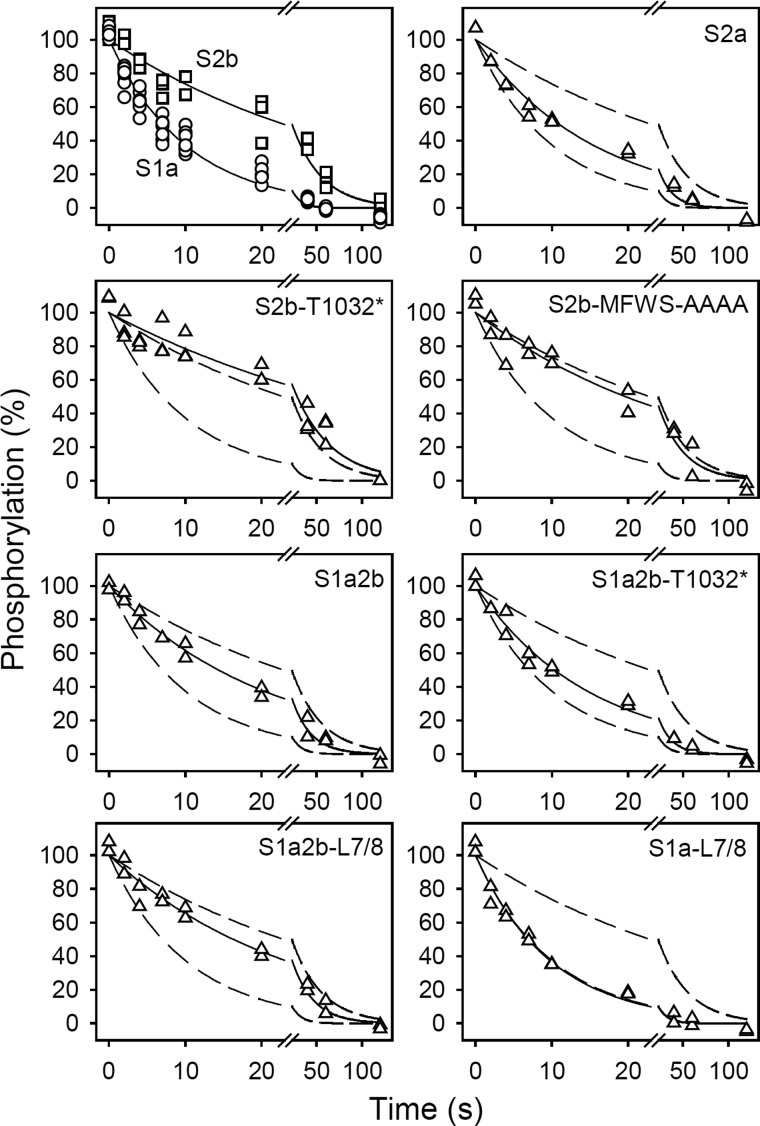
FIGURE 4.
Kinetics of E2P dephosphorylation. The microsomal enzyme was phosphorylated for 10 min at 25 °C in 100 mm MES/Tris (pH 6.0), 10 mm MgCl2, 2 mm EGTA, 30% (v/v) dimethyl sulfoxide (the organic solvent ensuring maximal amount of phosphoenzyme), and 0.5 mm 32Pi. Dephosphorylation was then studied at 0 °C by a 19-fold dilution of pre-chilled phosphorylated microsomes into ice-cold medium containing 40 mm MOPS/Tris (pH 7.0), 10 mm KCl, 2 mm MgCl2, 2 mm EGTA, and 0.5 mm nonradioactive Pi, followed by acid quenching at the indicated time intervals. All data points are shown. The lines show the best nonlinear regression fits of a monoexponential decay function, giving the rate constants listed in Table 2. In each case, the 100% value corresponds to the level of phosphoenzyme present just prior to the initiation of dephosphorylation. The broken lines represent the curves corresponding to wild type SERCA1a and SERCA2b from the upper left panel.