FIGURE 6.
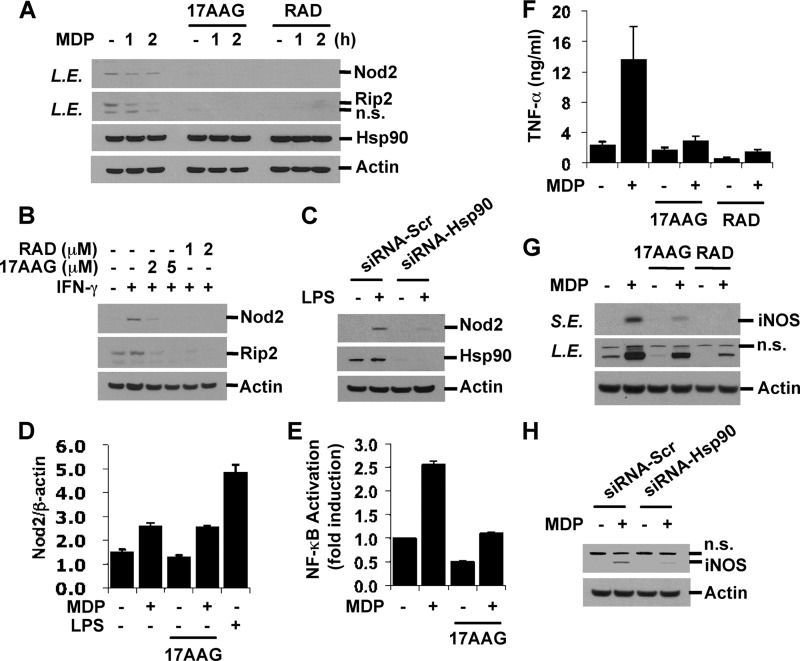
Hsp90 inhibition decreases endogenous and inducible levels of Nod2 and Rip2 and blocks MDP response. A and B, RAW264.7 cells were pretreated with 17AAG (2 or 5 μm) or RAD (1 or 2 μm) for 8 h and then stimulated with MDP (100 μg/ml) for the indicated times or IFN-γ (100 units/ml) for 6 h in the presence of Hsp90 inhibitors. C, RAW264.7 cells were transfected with siRNAs targeting Hsp90 or control siRNAs. Forty-eight hours after transfection, cells were stimulated with LPS (0.1 ng/ml) for 6 h. Cell extracts were subjected to Western blot analysis for Nod2, Rip2, Hsp90, and actin. D, RAW264.7 cells were pretreated with 17AAG (2 μm) and then stimulated with MDP for 5 h in the presence or absence of 17AAG. LPS (10 ng/ml; 5 h) was used as a positive control. E, HEK293T cells were co-transfected with pBVI-Luc reporter and pRLNull plasmids. Twenty-four hours after transfection, cells were co-treated with MDP and 17AAG (2 μm) for 20 h. NF-κB activity was measured by Dual-Luciferase assay. F and G, RAW264.7 cells were pretreated with 17AAG (2 μm) or RAD (1 μm) and then stimulated with MDP for 24 h. TNF-α in the media was measured by ELISA. Cell extracts were subjected to Western blot analysis for iNOS and actin. H, RAW264.7 cells were transfected as in C. Cells were stimulated with MDP for 24 h. The iNOS level was detected by Western blotting. Error bars represent the mean ± S.D. of triplicates. Data are representative of three independent experiments with similar results. L.E., long exposure; S.E., short exposure; n.s., nonspecific; Scr, scrambled.