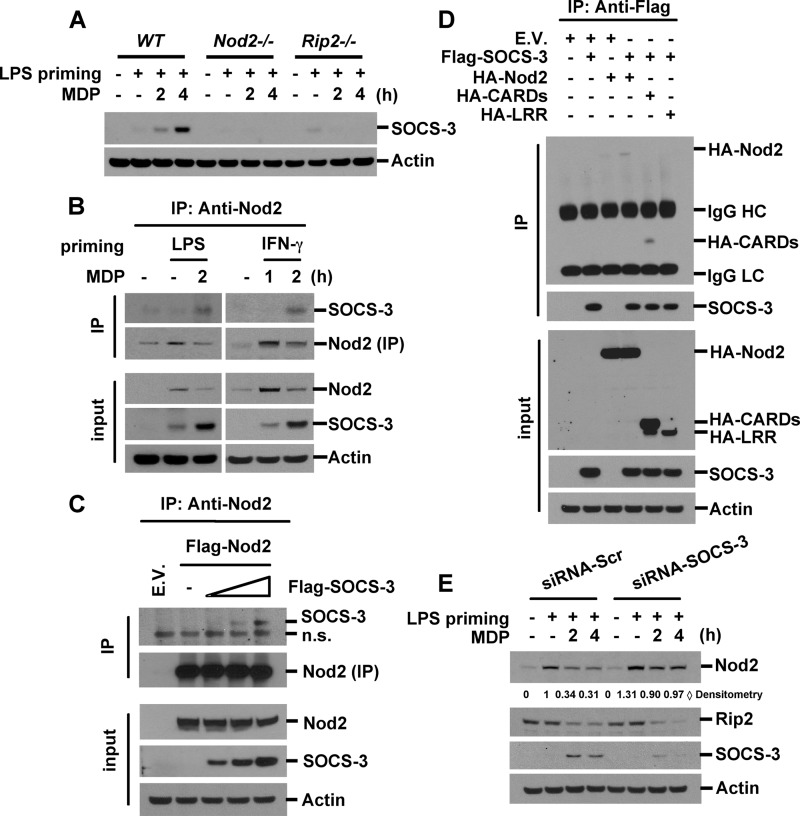
FIGURE 7.
Knockdown of SOCS-3 suppresses MDP-mediated Nod2 degradation. A, wild-type, Nod2-deficient, or Rip2-deficient BMDM were pretreated with LPS (0.2 ng/ml) for 6 h and then stimulated with MDP (100 μg/ml) for the indicated times. Cell extracts were subjected to Western blot analysis for SOCS-3 and actin. B, RAW264.7 cells were pretreated with LPS or IFN-γ for 6 h and then incubated with MDP for the indicated times. Nod2 was immunoprecipitated (IP) with anti-Nod2 antibody. Nod2-associated SOCS-3 was detected by Western blotting. C, HEK293T cells were transfected with FLAG-Nod2 and various concentrations of FLAG-SOCS-3 plasmids. Forty-eight hours after transfection, total cellular proteins were extracted. Cell extracts were immunoprecipitated with anti-Nod2 antibody. Co-precipitated proteins were analyzed by Western blotting with anti-SOCS-3 antibody. D, HEK293T cells were transfected with the expression vectors for FLAG-SOCS-3 and HA-Nod2 deletion mutants. SOCS-3 was immunoprecipitated with anti-FLAG antibody. SOCS-3-associated Nod2 deletion mutants were detected by Western blotting using anti-HA antibody. E, RAW264.7 cells were transfected with siRNAs for SOCS-3 and control siRNAs. Forty-eight hours after transfection, cells were treated with LPS (0.5 ng/ml) for 6 h and then stimulated with MDP for the indicated times. Cell extracts were subjected to Western blot analysis for Nod2, Rip2, SOCS-3, and actin. Data are representative of three independent experiments with similar results. n.s., nonspecific; E.V., empty vector; Scr, scrambled.