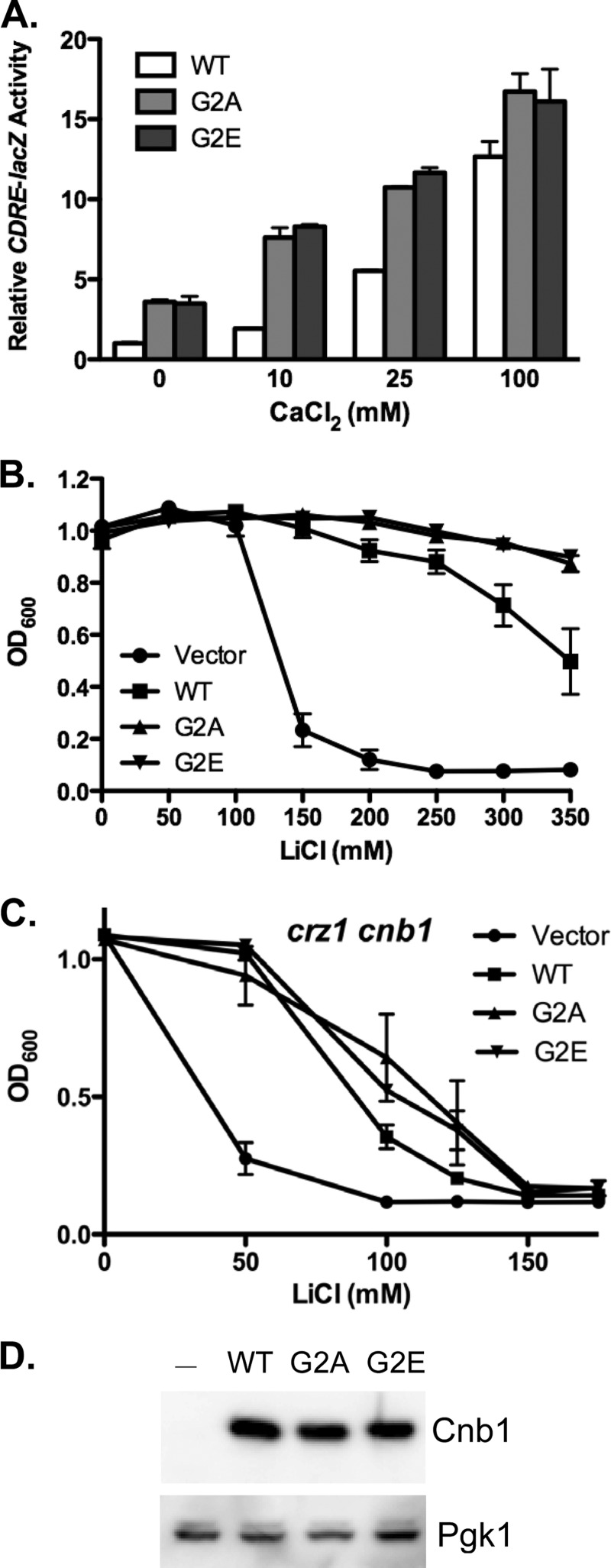
FIGURE 2.
Cnb1 myristoylation antagonizes calcineurin activation. The myristoylated glycine residue of Cnb1 was mutated to either alanine (Cnb1-G2A) or glutamic acid (Cnb1-G2E). Mutants were then tested for their ability to restore calcineurin-mediated gene expression and ion-resistant growth to cnb1Δ yeast. A, Cnb1Δ yeast harboring the CDRE-lacZ reporter gene were transformed with plasmids expressing Cnb1, Cnb1-G2A, or Cnb1-G2E. Yeast growing in YPD (pH 5.5) were stimulated for 4 h by the addition of extracellular CaCl2 prior to harvesting for quantitative β-galactosidase assays. Data plotted are average of four independent yeast transformants ± S.D. B and C, ion tolerance assays. cnb1Δ (B) or cnb1Δ crz1Δ (C) yeast were transformed with Cnb1, Cnb1-G2A, Cnb1-G2E, or empty vector as indicated. Yeast were inoculated into YPD containing increasing concentrations of LiCl (in mm) and grown at 30 °C for 2 days. Growth was quantitated by measuring the A600. Data points are average of three independent yeast transformants assayed in parallel ± S.E. D, anti-Cnb1 Western blot analysis of protein extracts prepared from cnb1Δ yeast transformed with wild type Cnb1, Cnb1-G2A, Cnb1-G2E, or empty vector. Anti-Pgk1 was used for loading control comparison.