FIGURE 7.
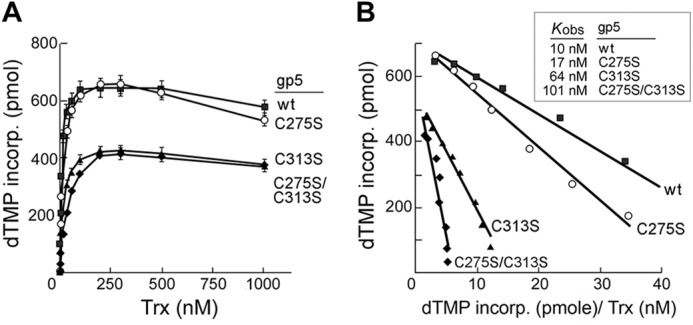
Binding affinity of wild-type gp5 and altered gp5 to trx. A, dependence of trx concentration on initial rate of DNA polymerase activity of gp5/trx is shown. Reactions (20 μl) contained 5 nm concentrations of the indicated gp5, 50 mm Tris-HCl, pH 7.5, 5 mm DTT, 50 mm NaCl, 10 mm MgCl2, 250 μm each of dGTP, dATP, dCTP, and [3H]TTP (10 cpm/pmol), 200 nm dA350/dT25 substrate. Increasing amounts of trx was added to each reaction as indicated. Reactions were incubated at 25 °C. The amount of DNA synthesis for each reaction was determined by measuring the amount of [3H]dTMP incorporated over 1 min as described under “Experimental Procedures.” B, shown is the determination of the observed equilibrium binding constant, Kobs. The data shown in A were used to generate Scatchard plots of initial rates versus ratio of initial rates to added concentration of trx. Kobs is equal to the negative slope of the corresponding curve. ■, wt gp5; ○, gp5-C275S; ▴, gp5-C313S; ♦, gp5-C275S/C313S.
