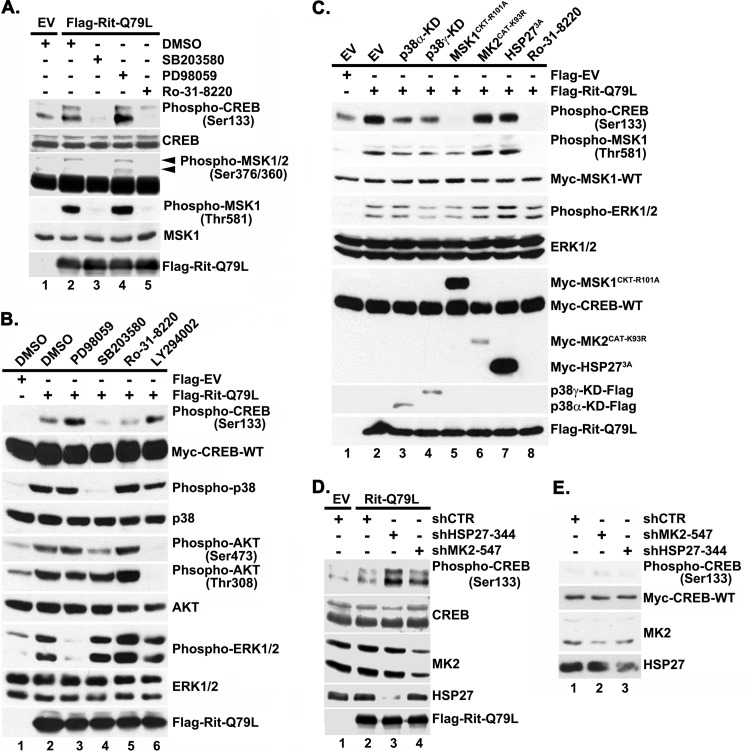
FIGURE 3.
Rit-mediated CREB activation requires p38-MSK1/2 signaling. A, Rit activates MSK1/2 and CREB in a p38-dependent manner. PC6 cells were pretreated with p38 (SB203580, 10 μm), MEK1/2 (PD98059, 10 μm), or MSK1/2 (Ro-31–8220, 2 μm) kinase inhibitors prior to transfection with Flag-RitQ79L (2 μg). Flag-EV transfection was used as control. Cell lysates were prepared after starvation (5 h) in the presence of appropriate inhibitors, and subjected to phospho-specific immunoblotting. The results are representative of three separate experiments. B, Rit-mediated CREB phosphorylation does not rely upon PI3 kinase. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L after pretreatment with SB203580 (10 μm), PD98059 (10 μm), Ro-31–8220 (2 μm), LY294002 (10 μm), or vehicle (DMSO) as control, and cell lysates subjected to phospho-specific immunoblotting. C, CREB activation is inhibited by DN-p38 or DN-MSK1, but not DN-MK2 or DN-HSP27 expression. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) together with DN-mutants of p38α-KD, p38γ-KD, MSK1CKT-R101A, MK2CAT-K93R, or HSP273A (0.5 μg). MSK1/2 inhibitor Ro-31-8220 (2 μm, 30 min) pretreatment was used as control. Cell lysates were examined with phospho-specific immunoblotting. Results are representative of four independent experiments. D, MK2 and HSP27 silencing does not inhibit Rit-mediated CREB phosphorylation. PC6 cells were transfected with either Flag-EV or Flag-RitQ79L (1.5 μg) in the presence or absence of shCTR, shMK2–547 or shHSP27–344 (1.5 μg) and subjected to G418 (400 μg/ml) selection. 60 h after transfection, cell lysates were prepared and phosphorylated CREBSer133 levels examined by immunoblotting. Results are representative of three independent experiments. E, PC6 cells were transfected with shCTR, shMK2–547, or shHSP27–344 (1.5 μg) and subjected to G418 selection as in D. Cell lysates were prepared and phosphorylated CREBSer133 levels examined by immunoblotting. Note that MK2 and HSP27 silencing does not stimulate CREB phosphorylation.