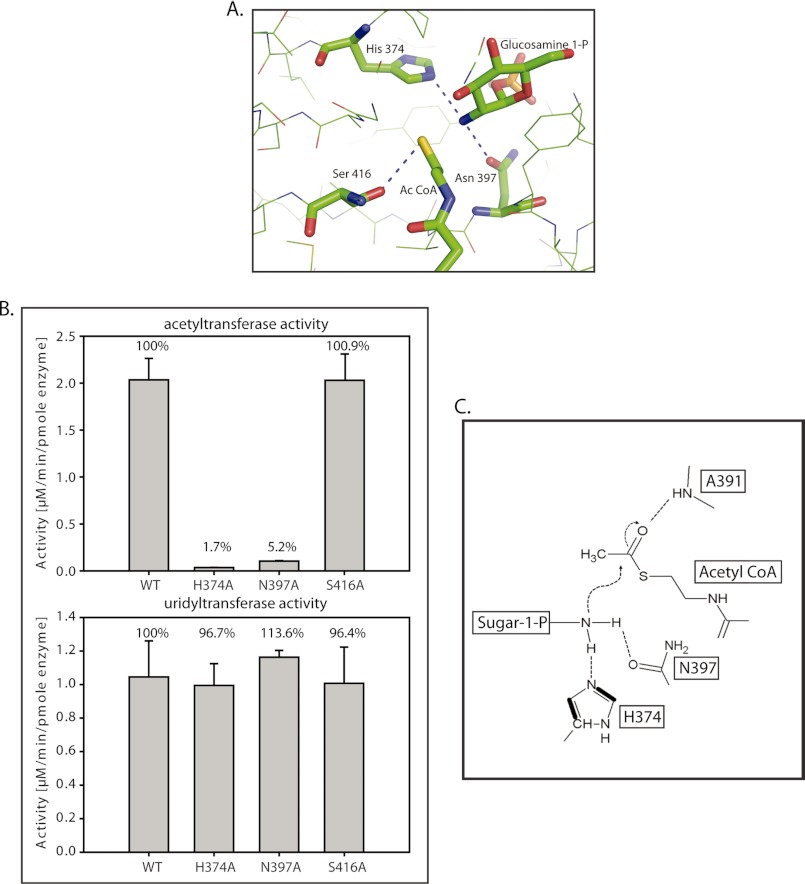
FIGURE 3.
Residues participating in the acetyl transfer reaction. A, the acetyltransferase active site of GlmUMtb reveals probable catalytic residues His-374, Asn-397, and Ser-416, which are highly conserved. Backbone amide of Ala-391 that interacts with the acetyl group is not shown. B, active site mutants were generated, and their acetyltransferase activities were assayed. The activities of H374A and N397A are almost abolished. However, the activity of S416A is not affected significantly. Uridyltransferase activities of the proteins were used as a control. C, a schematic of the proposed acetyltransferase reaction mechanism in GlmUMtb (see text). The amino group activated by His-374 and Asn-397 launches a nucleophilic attack on the carbonyl carbon of acetyl-CoA. Simultaneously, the resultant negative charge on the carbonyl oxygen (oxyanion) is stabilized by the amide backbone of Ala-391.