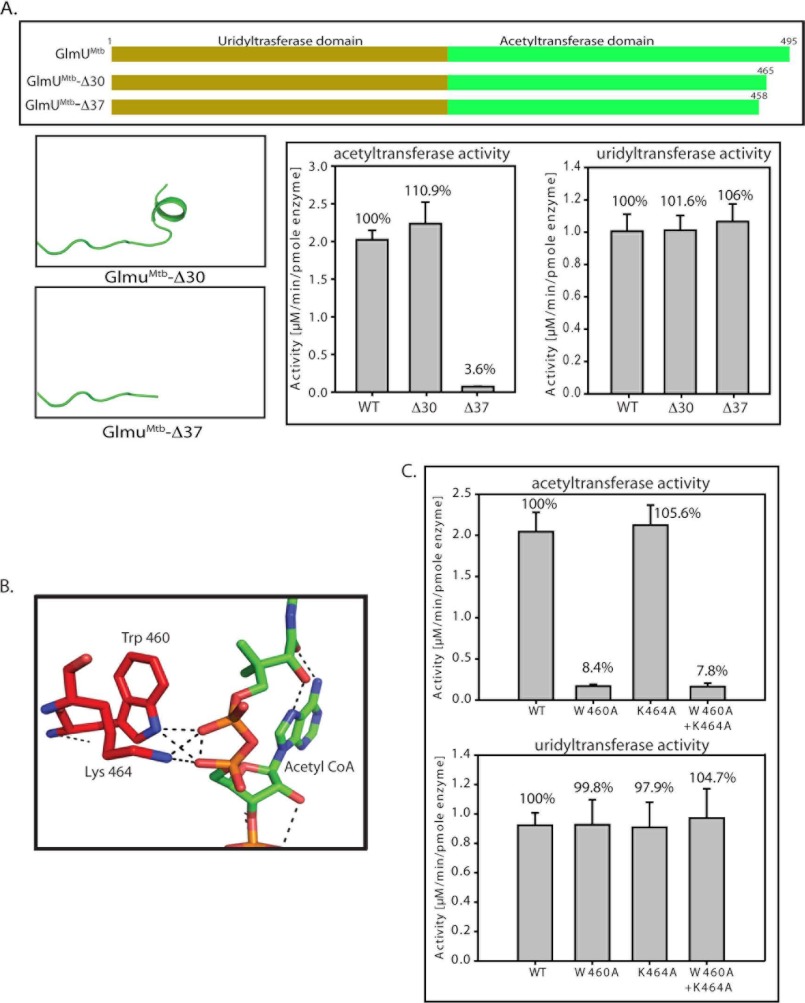
FIGURE 5.
Tail truncation experiments identify residues important for acetyltransferase activity. A, acetyltransferase assays show that a complete loss in activity is seen only for Δ37, whereas Δ30 shows an activity comparable with the wild type protein. The right panel depicts the unchanged uridyltransferase activities for the mutants. B, structure analysis of GlmUMtb(AcCoA) structure revealed that Trp-460 and Lys-464 lie in this region and provide important interactions with the backbone phosphate of acetyl-CoA. C, W460A mutant displays complete loss in acetyltransferase activity, whereas K464A does not. The loss in activity of the double mutant (W460A + K464A) reinforces the importance of Trp-460. The control uridyltransferase assays are shown on the right.