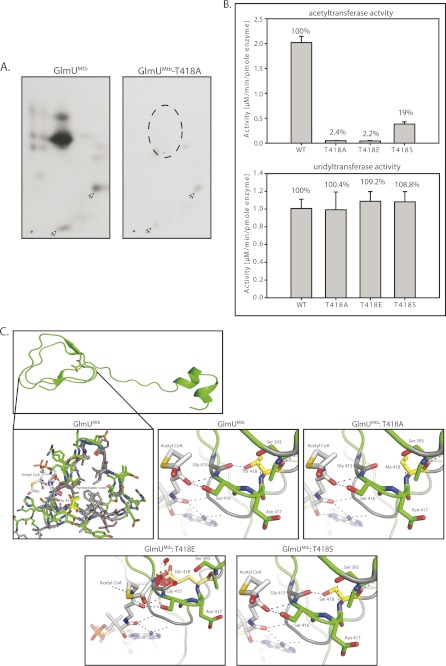
FIGURE 7.
The effect of phosphorylation of GlmUMtb by PknB. A, in vitro-phosphorylated GlmUMtb or mutants were digested with trypsin and the resulting phosphopeptides were mapped by two-dimensional resolution on thin layer chromatography. B, Thr-418 was mutated to glutamate (phospho-Thr mimic) and to alanine (phosphorylation negative). Acetyltransferase activities of GlmUMtb-WT and the phospohomutants are shown in the left panel. Corresponding uridyltransferase activities are shown in the right panel. C, the acetyltransferase domain, which consists of the β-helix, has a highly hydrophobic core (gray residues) and a few polar residues. Thr-418 is shown in yellow. Hydroxyl group of Thr-418 makes a hydrogen bond with the backbone oxygen of Gly-415. Mutation of Thr-418 to alanine (T418A) would disrupt this interaction (black arrow), whereas mutation to a glutamate (T418E) would introduce steric clashes in the region as shown by red patches. However, mutation to serine (T418S) would preserve the polar interaction with the backbone of Gly-415. The hydroxyl and backbone amine group of Ser-416 interacts with the backbone oxygen of the acetyl-CoA. It is expected that perturbing these interactions would affect the positioning of the acetyl group for catalysis.