FIGURE 6.
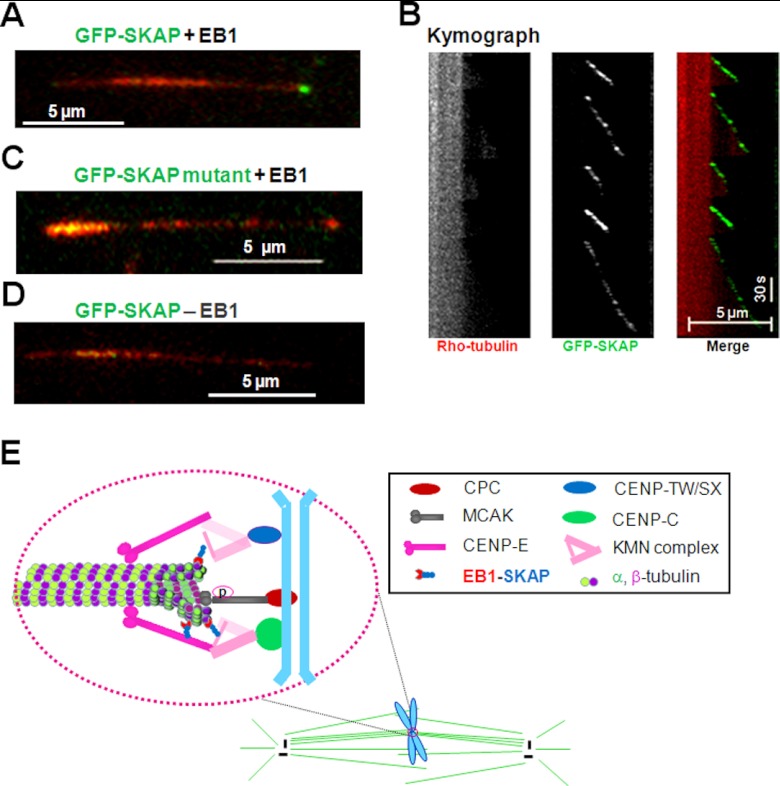
SKAP constitutes a dynamic interface between KMN and growing microtubule plus-ends. A, TIRFM analysis shows that SKAP tracks microtubule plus-ends. The growing microtubule plus-end tracking of SKAP depends on EB1 in vitro. B, the corresponding kymograph shows that SKAP orchestrates the microtubule dynamics because SKAP is specifically located in the growing ends. C, validation of plus-end tracking of SKAP depends on its EB1-binding motif SXLP because mutation of LP into NN abolishes its tracking and end binding activity in vitro. D, validation of plus-end tracking of SKAP depends on EB1 because the absence of EB1 fails to support SKAP tracking activity in vitro. E, the faithful progression of mitosis is orchestrated by dynamic interaction between spindle microtubules and the kinetochore; it ensures the inheritance of genetic materials. The conserved KMN network, including KNL-1, Mis12 complex, and Ndc80 complex, constitutes the core microtubule binding site of the kinetochore. The CENP-C·CENP-T complex helps to target KMN to the centromere. The direct binding of SKAP to Mis13 and plus-end tracking protein EB1 links the outer kinetochore to microtubule plus-ends. Together with some other proteins, such as CENP-E, CLASP1, and MCAK, SKAP modulates kinetochore-microtubule attachment, sister kinetochore oscillations, and microtubule dynamics and regulates spindle plasticity.