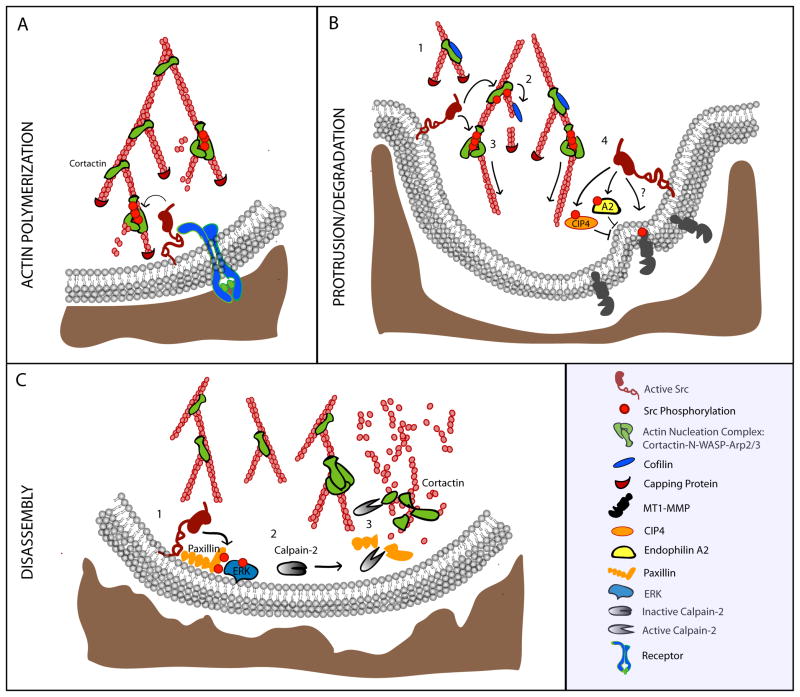
Figure 3. Src regulation of invadosomes.
(A) Src becomes activated downstream of receptor and/or integrin-mediated signaling and phosphorylates multiple substrates to induce actin polymerization. Cortactin is phosphorylated at three tyrosine residues and interacts with WASP and Arp2/3 to nucleate new actin branches. (B) 1: New actin branches are stabilized by dephosphorylated cortactin which binds cofilin in an inactive state. 2: During later stages, cortactin is phosphorylated and cofilin is released. Cofilin cleaves actin filaments resulting in the formation of new barbed ends. 3: Cortactin interacts with the N-WASP/Arp2/3 complex to nucleate de novo actin branches. 4: Src can regulate MT1-MMP matrix degradation activity by phosphorylating the cytoplasmic tail at Y573 which may regulate its trafficking. Alternatively, Src can phosphorylate endophilin A2 or the Cdc42 interacting protein (CIP4) to inhibit endocytosis of MT1-MMP thereby increasing surface expression. (C) During disassembly, Src phosphorylates paxillin which activates Erk signaling. Erk leads to the activation of the protease Calpain-2 which cleaves multiple invadosome proteins including paxillin and cortactin to mediate invadosome disassembly.