Abstract
Purpose
To determine the effects of total body irradiation (TBI) dose, fractionation, and lung shielding on hematopoietic stem cell homing to the bone marrow.
Material and Methods
Bone marrow (BM) cells were extracted from tibiae and femurs of B6-GFP mice and were transplanted into B6 mice. Recipient mice had either: 1) no radiation, 2) single dose TBI at 13.6 Gray (Gy), 3) single dose TBI at 13.6 Gy with reduced lung exposure to 0.4 Gy by shielding, 4) split dose TBI at 12 Gy to twice/day over four days, or 5) split dose TBI at 12 Gy to twice/day over four days with reduced lung exposure to 0.36 Gy by shielding. The last radiation exposure preceded tail vein injection by 4–6 hours. Mice were sacrificed after 18 hours.
Results
Homing of GFP positive, lineage negative cells was not significantly improved in any irradiated group compared to control. Homing of GFP positive, lineage negative, Kit positive cells was significantly worse in all irradiated groups.
Conclusion
TBI does not improve the homing of lineage negative donor BM cells to the recipient marrow. Homing of lineage negative, Kit positive donor BM cells was significantly worse following TBI, with or without lung dose reduction.
Keywords: TBI, Lung Shielding, Dose Reduced, BID, Stem
Introduction
Bone marrow (BM) or peripheral blood stem cell transplantation (SCT) can be curative in the treatment of various human malignancies[1–5]. In order for SCT to be successful, the transplanted stem cells must home to the correct location within the BM [6–8]. In mouse models, irradiation has been found to alter the homing of stem cells[9–11].
In humans, evidence suggests that total body irradiation (TBI) improves post-transplant survival compared to the use of chemotherapy alone[12–14]. Improved survival, however, may be mitigated by increased toxicity. In particular, lung toxicity has been shown to impact survival[15–17]. One strategy to reduce pulmonary toxicity is to simply reduce the lung dose directly by utilizing lung shielding[18]. Several studies have described an association between lung dose reduction, reduced pulmonary-related mortality and improved overall survival[19–21].
Specifically our clinical experience showed that, compared to twice daily radiation alone, twice daily, lung dose reduced TBI can improve survival in a subset of patients[19]. Further analysis showed that this observed survival benefit of lung radiation dose reduction did not result from improved lung function[22].
It is known that TBI can alter homing of stem cells[9–11]. We wondered if the observed survival benefit of twice daily, TBI with lung radiation dose reduction may result from altered homing of stem cells to the bone marrow. To answer this question, mouse experiments were performed to test the effect of twice daily, lung dose reduced TBI on homing of hematopoietic stem and progenitor cells to recipient bone marrow.
Materials and Methods
Animals
Normal C57BL/6 (B6) and the B6-EGFP transgenic mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and were maintained at NIH animal facilities under normal care and nutrition conditions. The EGFP mice have been backcrossed to B6 for 15 generations to ensure a relatively pure B6 genetic background. Similar combinations were used by others in studying hematopoietic cell and niche interactions[23]. All mice were used at 2–6 months of age and only male mice were used in the current study. All experiments were carried out under the aegis of a protocol approved by the National Cancer Institute Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animal Resource, (1996) National Research Council.
TBI and Lung Shielding
To specifically study the effects of lung shielding, we used a Therapax DXT300 X-ray irradiator (Precision X-ray, Inc., North Branford, CT) using 2.0 mm Al Filtration (300 KVp) to irradiate B6 recipients at a dose rate of 2.35 Gy/min. We divided 25 B6 recipients into five groups of five mice each: 1) CON: No irradiation control, 2) SD-NS: 13.6 Gray (Gy), single dose TBI without lung shielding, 3) SD-BLS: 13.6 Gy single dose TBI with bilateral lung shielding, 4) BID-BLS: 12 Gy TBI in fractionated dose with bilateral lung shielding, 5) BID-NS: 12 Gy TBI in fractionated doses without lung shielding. Lung shielding consisted of a 5 half-value layer block which reduced transmission to approximately 3% of the given dose. Fractionation, similar to the treatment of our clinical human cohort, consisted of twice daily radiation, 4 hours apart at 1.5 Gy each time over 4 days for a total of 12 Gy. In this experiment, each recipient received 14.0 × 106 EGFP BM cells.
BM cells from EGFP donors were at 28 × 106 cells/mL in IMDM. Each recipient mouse received 0.5 mL donor cells which were delivered through tail vein injection at 4–6 hours following the last radiation treatment. Recipient mice were euthanized 18 hours after cell injection and BM cells were collected and analyzed for donor hematopoietic cell homing.
FACS Analysis and Detection of Hematopoietic Cell Homing in Recipient Mice
BM cells were extracted from bilateral femurs and tibiae of recipient mice 18 hours after cell injection, and were filtered through a 90 µm nylon mesh. Cells were counted to calculate total BM cell recovery. All cells were stained with an antibody cocktail containing: Lin (CD3, CD4, CD8, CD11b, CD45R, Gr1, Ter119)-PE + CD34-PE-Cy5 + CD117-APC. Proportion and total number of GFP cells in each cell fraction were calculated to compute cell recovery (homing efficiency).
Procedures for flow cytometry were adapted as previously described[24]. In brief, BM cells were incubated in Geys solution (130.68 mM NH4Cl, 4.96 mM KCl, 0.82 mM Na2HPO4, 0.16 mM KH2PO4, 5.55 mM Dextrose, 1.03 mM MgCl 2, 0.28 mM MgSO4, 1.53 mM CaCl2 and 13.39 mM NaHCO3) for ten minutes on ice to lyse RBCs. After washing with a flow buffer (2.68 mM KCl, 1.62 mM Na2HPO4, 1.47 mM KH2PO4, 137 mM NaCl, 7.69 mM NaN3, and 1% BSA), cells were incubated with antibody mixtures on ice for 30 minutes. Monoclonal antibodies for mouse CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), CD11b (clone M1/70), CD45R (B220, clone RA3-6B2), CD117 (c-Kit, clone 2B8), erythroid cells (clone Ter119), granulocytes (Gr1/Ly6-G, clone RB6-8C5), and stem cell antigen 1 (Sca1, clone E13-161) were all from BD Biosciences (San Diego, CA). Stained cells were analyzed on an LSRII flow cytometer using the FACSDiva software (Becton Dickinson, San Jose, CA).
Statistics
Proportions of EGFP+ and EGFP+Lin−CD117+ cells in BM were multiplied by the total number of BM cells to calculate the total number of donor cells recovered in recipient BM as a percentage of EGFP+ and EGFP+Lin−CD117+ cell homing to recipient BM. Total number of BM cells per mouse was estimated assuming that one femur and one tibia contain 12.5% of total BM cells[25].
Data were analyzed using JMP Statistical Discovery software (SAS Institute Inc., Cary, NC), and were presented as mean with standard errors. A P value <0.05 was considered as statistically significant.
Results
Radiation Reduces Hematopoietic Cell Homing to Host BM
Lung shielding did not improve homing of any subset of GFP positive cells to the BM of recipient mice following either the once daily or fractionated TBI
To test the effect of lung shielding, we used X-rays to irradiate recipient mice as shown in Figure 1 with the lung-shielding shown in Figure 1B. The EGFP donor mice we used in the current study had higher levels of EGFP transgene expression in BM cells.
Figure 1. Mouse Total Body Irradiation and Lung Shielding.
A Therapax DXT300 X-ray irradiator was used as the source of irradiation for all recipient mice used in the current study. Mice were anesthetized and place on a specifically designed table. The effective X-ray area can accommodate four mice each time. Mice were irradiated for the entire body without shielding (A), or with lead shielding to reduce lung exposure to 3% of the effective dose (B).
The top, left panel of Figure 2 demonstrates that 4% of recovered cells were lineage negative. The top right panel shows that 31% of the lineage negative cells were also Kit+. The lower 2 panels show that 98% of Lin− cells and 99% of Lin−Kit+ BM cells from EGFP donor mice were positive for EGFP expression, the marker used to track cells in the recipient BM.
Figure 2. Flow Cytometric Analysis of Donor Bone Marrow Cells.
All recipient mice were then injected with BM cells from B6-EGFP donors for which the vast majority of Lin− (98%) and Lin−Kit+ (99%) of donor BM cells were EGFP+.
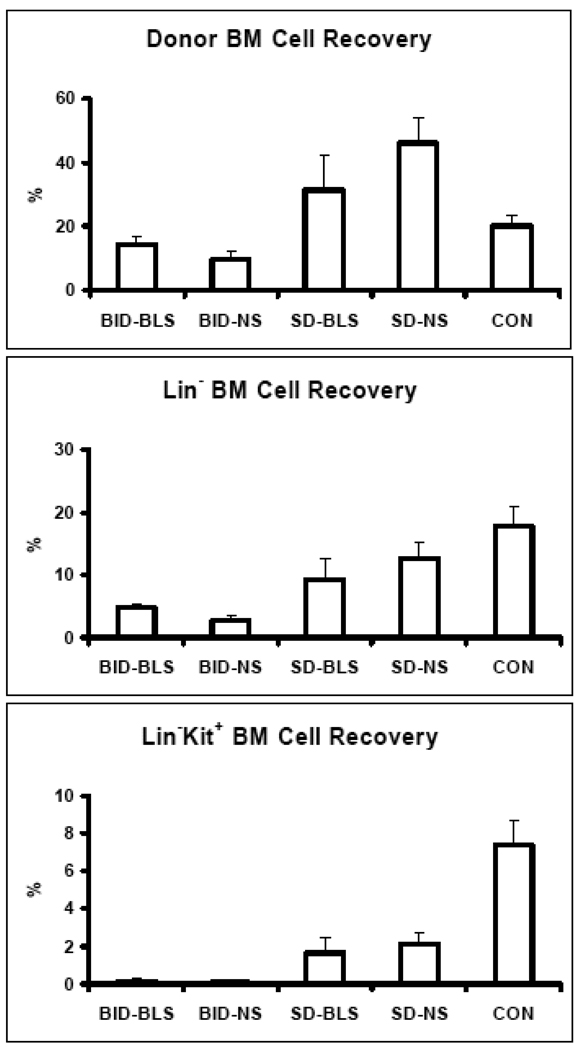
Homing of total GFP positive BM cells (Figure 3, top), lineage negative GFP positive BM cells (Figure 3, middle), and lineage negative Kit positive GFP positive BM cells (Figure 3, bottom panel) was not significantly improved in any irradiated group compared to control. Homing of GFP positive, lineage negative cells was not improved by single dose or fractionated TBI. In fact, fractionated radiation therapy showed a 2 to 5 fold decrease in donor hematopoietic cell homing compared to single dose radiation (Figure 3).
Figure 3. Effect of Fraction Dose and Lung Shielding on Hematopoietic Cell Homing.
Homing of EGFP donor Lin−Kit+ cell to recipient BM was shown as percentages of total BM (top), Lin− (middle) and Lin−Kit+ (bottom) cell recovery in recipient BM were computed and presented as means with standard error bars respectively, showing that irradiation reduces hematopoietic cell homing to recipient BM and fractionated dose further reduces cell homing, with or without lung shielding. CON = No irradiation control. SD-NS = 13.6 Gray (Gy), single dose TBI without lung shielding. SD-BLS = 13.6 Gy single dose TBI with bilateral lung shielding. BID-BLS= 12 Gy TBI in fractionated dose with bilateral lung shielding. BID-NS =12 Gy TBI.
As shown in the bottom panel of Figure 3, homing of GFP positive, lineage negative, Kit positive cells was significantly worse in all irradiated groups. Fractionated radiation therapy showed a 5 fold decrease in homing compared to single dose radiation. With either single dose or fractionated doses, lung shielding showed no beneficial effect for donor hematopoietic cells homing to recipient BM.
Discussion
This is the first study to show that lung shielding does not significantly alter homing of transplanted stem cells following TBI. Moreover, this study shows that twice daily, fractionated TBI does not improve the homing of donor BM cells to the recipient marrow. Homing of lineage negative, and of the subset of lineage negative, Kit positive donor BM cells, was actually significantly worse following the twice daily over 4 day TBI regimen.
Though the true hematopoietic stem and progenitor cells has yet to be fully defined, multiple studies do show that transplanted cells capable of reconstituting hematopoesis in lethally irradiated mice are lineage negative and Kit positive[25–28]. Cao et al. showed that such transplanted stem cells can be later recovered from the femurs[25]. Moreover, Cao et al showed that it takes weeks for substantial proliferation to occur. In an MR imaging study, Daldrup-Link et al. showed that the majority of stem cell homing to the marrow occurs within 4–24 hours following injection[29]. As in our study, Kimura et al. also used an 18 hour time point to test the homing of stem cells without any substantial proliferation[30].
This study was designed to accentuate any radiation dose or fractionation induced differences in the homing of lineage negative, Kit positive donor BM cells between groups by using a higher total dose in the single fraction arms (13.6 Gy versus 12 Gy) and by limiting lung dose to 3% (compared to 50% in the clinical setting) in the lung shielding groups.
Despite these attempts to accentuate differences which favored the fractionated arms, the fact that fractionated radiation therapy caused a decrease in donor hematopoietic cell homing compared to single dose radiation is a fresh observation for which we lack a definitive explanation. Kovacs et al. reported that fractionated radiation therapy generates two forms of dose-dependent damage in the marrow. In the first form of dose dependent damage, an early lesion arises in the blood-forming CAFC subpopulations. In the second form of dose dependent damage, a delayed lesion arises which involves the persistent expression of a dysfunctional microenvironmental phenotype[31]. Based on this data, we speculate that fractionated doses of irradiation may have produced more damage to host stromal environment than single dose irradiation thus, hampering the homing of infused donor hematopoietic stem and progenitor cells.
Of note, the dose rate of 2.35 Gy/minute used in this study was different from the clinical dose rate of 0.12 Gy/minute. However, Colis et al. showed that TBI dose rate, varying from 0.01 to 5.85 Gy/minute, did not impact homing of stem cells to the bone marrow[10].
Our data showing decreased hematopoietic stem and progenitor cell homing after TBI echo Collis et al. who showed an approximately five fold reduction in lineage negative hematopoietic cell homing to the BM compared to unirradiated control animals[10]. Similarly, comparing radiated to non-radiated mice, Plett et al found a 5 to 30 fold reduction in homing of stem cells capable of long-term hematopoiesis in secondary recipients[11].
Conversely, ours and the aforementioned data contravene the experience of Bastianutto et al. who found that local irradiation does induce a four fold greater homing of hematopoietic stem cells to the locally irradiated BM[32]. In this experiment, however, TBI was not performed in any group. Therefore, it remains possible that the effects of isolated local irradiation are quite different from TBI in the induction of hematopoietic stem cell homing.
Overall, multiple publications and our data support decreased hematopoietic stem and progenitor cell homing after TBI[10, 11]. However, this data must be interpreted with caution. Human data from our group and others suggests that the stem cell dose is not linearly related to survival. In fact, many series have shown that above an optimum stem cell dose (approximately 8–10 × 106 cells/kg) the probability of mortality actually increases with increasing stem cell dose[33–35]. Given this fact that (above an optimum level) increased stem cell dose actually decreases survival, one cannot simply assume that the decreased stem cell homing seen following TBI will necessarily diminish survival.
Multiple questions remain about the homing of lineage negative, Kit positive cells following TBI. Previous studies have shown that some fraction of transplanted stem cells do home to spleen[36, 37] [38, 39] and/or lung[36, 39]. Therefore, one may posit that TBI with or without lung shielding may increase homing to these other sites. However a prior iteration of these experiments, similar to those detailed here but with female mice (data not shown,) showed consistently less homing -- on a similar scale as shown for BM-- of GFP positive, lineage negative, Kit positive cells to spleen, lung, and BM following TBI with or without lung shielding. Thus, it appears that TBI, with or without lung shielding, decreases homing of lineage negative, Kit positive cells to BM, spleen and lung.
Future Directions
Stem cell transplant is a complicated process with many effective approaches (eg. types chemotherapy used, done with or without TBI) and a multitude of variations (eg. dose of chemotherapy used, single fraction or multiple fraction TBI with or without lung dose reduction, low dose.) The absence of a definitively superior approach suggests that no optimum regimen will soon emerge. Therefore, each approach will need to optimized. Given the complexity of the successful transplant, we advocate making only those limited changes to the transplant regimen necessary to test the hypothesis while holding all other variables constant.
We have found no pre-clinical evidence that TBI with bilateral lung shielding improves stem cell homing. The mechanisms which explain the survival benefit seen in a subset of patients treated with lung dose reduced, twice daily TBI remain unknown[19]. However, given the success of lung dose reduction and absence of any homing advantage in preclinical models, we are pursuing lower doses of TBI in selected older patients.
Conclusion
The current data adds to the existing literature by showing that the survival benefit seen with lung dose reduced, twice daily TBI[19] likely does not arise from improved homing of hematopoietic stem and progenitor cell homing to the BM. However, no definite conclusion can be reached about homing of the true stem cell subset until that subset is adequately defined.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Financial Disclosure Statement:
The authors have no financial interests to disclose.
References
- 1.Huisman C, Meijer E, Petersen EJ, Lokhorst HM, Verdonck LF. Hematopoietic stem cell transplantation after reduced intensity conditioning in acute myelogenous leukemia patients older than 40 years. Biol Blood Marrow Transplant. 2008 Feb;14(2):181–186. doi: 10.1016/j.bbmt.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin's lymphoma. J Clin Oncol. 2008 Jan 10;26(2):211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 3.Inamoto Y, Suzuki R, Kuwatsuka Y, Yasuda T, Takahashi T, Tsujimura A, et al. Long-term outcome after bone marrow transplantation for aplastic anemia using cyclophosphamide and total lymphoid irradiation as conditioning regimen. Biol Blood Marrow Transplant. 2008 Jan;14(1):43–49. doi: 10.1016/j.bbmt.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. The New England journal of medicine. 2007 Mar 15;356(11):1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 5.Das-Gupta EP, Russell NH, Shaw BE, Pearce RM, Byrne JL. Long-term outcome of unrelated donor transplantation for AML using myeloablative conditioning incorporating pretransplant Alemtuzumab. Biol Blood Marrow Transplant. 2007 Jun;13(6):724–733. doi: 10.1016/j.bbmt.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends in molecular medicine. 2007 Feb;13(2):72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Chute JP. Stem cell homing. Current opinion in hematology. 2006 Nov;13(6):399–406. doi: 10.1097/01.moh.0000245698.62511.3d. [DOI] [PubMed] [Google Scholar]
- 8.Quesenberry PJ, Colvin G, Abedi M. Perspective: fundamental and clinical concepts on stem cell homing and engraftment: a journey to niches and beyond. Experimental hematology. 2005 Jan;33(1):9–19. doi: 10.1016/j.exphem.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Francois S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem cells (Dayton, Ohio) 2006 Apr;24(4):1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 10.Collis SJ, Neutzel S, Thompson TL, Swartz MJ, Dillehay LE, Collector MI, et al. Hematopoietic progenitor stem cell homing in mice lethally irradiated with ionizing radiation at differing dose rates. Radiation research. 2004 Jul;162(1):48–55. doi: 10.1667/rr3197. [DOI] [PubMed] [Google Scholar]
- 11.Plett PA, Frankovitz SM, Orschell-Traycoff CM. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 2002 Nov 15;100(10):3545–3552. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- 12.Blaise D, Maraninchi D, Michallet M, Reiffers J, Jouet JP, Milpied N, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001 Jun 1;97(11):3669–3671. doi: 10.1182/blood.v97.11.3669. [DOI] [PubMed] [Google Scholar]
- 13.Dusenbery KE, Daniels KA, McClure JS, McGlave PB, Ramsay NK, Blazar BR, et al. Randomized comparison of cyclophosphamide-total body irradiation versus busulfan-cyclophosphamide conditioning in autologous bone marrow transplantation for acute myeloid leukemia. Int J Radiat Oncol Biol Phys. 1995 Jan 1;31(1):119–128. doi: 10.1016/0360-3016(94)00335-i. [DOI] [PubMed] [Google Scholar]
- 14.Ringden O, Labopin M, Tura S, Arcese W, Iriondo A, Zittoun R, et al. A comparison of busulphan versus total body irradiation combined with cyclophosphamide as conditioning for autograft or allograft bone marrow transplantation in patients with acute leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) British journal of haematology. 1996 Jun;93(3):637–645. doi: 10.1046/j.1365-2141.1996.d01-1681.x. [DOI] [PubMed] [Google Scholar]
- 15.Della Volpe A, Ferreri AJ, Annaloro C, Mangili P, Rosso A, Calandrino R, et al. Lethal pulmonary complications significantly correlate with individually assessed mean lung dose in patients with hematologic malignancies treated with total body irradiation. Int J Radiat Oncol Biol Phys. 2002 Feb 1;52(2):483–488. doi: 10.1016/s0360-3016(01)02589-5. [DOI] [PubMed] [Google Scholar]
- 16.Onishi Y, Mori S, Kusumoto S, Sugimoto K, Akahane D, Morita-Hoshi Y, et al. Unrelated-donor bone marrow transplantation with a conditioning regimen including fludarabine, busulfan, and 4 Gy total body irradiation. Int J Hematol. 2007 Apr;85(3):256–263. doi: 10.1532/IJH97.06199. [DOI] [PubMed] [Google Scholar]
- 17.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006 Oct;12(10):1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Labar B, Bogdanic V, Nemet D, Mrsic M, Vrtar M, Grgic-Markulin L, et al. Total body irradiation with or without lung shielding for allogeneic bone marrow transplantation. Bone Marrow Transplant. 1992 May;9(5):343–347. [PubMed] [Google Scholar]
- 19.Singh AK, Karimpour SE, Savani BN, Guion P, Hope AJ, Mansueti JR, et al. Pretransplant pulmonary function tests predict risk of mortality following fractionated total body irradiation and allogeneic peripheral blood stem cell transplant. Int J Radiat Oncol Biol Phys. 2006 Oct 1;66(2):520–527. doi: 10.1016/j.ijrobp.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest. 1992 May;101(5):1257–1264. doi: 10.1378/chest.101.5.1257. [DOI] [PubMed] [Google Scholar]
- 21.Carlson K, Backlund L, Smedmyr B, Oberg G, Simonsson B. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant. 1994 Nov;14(5):805–811. [PubMed] [Google Scholar]
- 22.Soule BP, Simone NL, Savani BN, Ning H, Albert PS, Barrett AJ, et al. Pulmonary function following total body irradiation (with or without lung shielding) and allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. 2007 Sep;40(6):573–578. doi: 10.1038/sj.bmt.1705771. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009 Jan 1;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, et al. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J Immunol. 2007 Apr 1;178(7):4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 25.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004 Jan 6;101(1):221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 27.Eto T, Winkler I, Purton LE, Levesque JP. Contrasting effects of P-selectin and E-selectin on the differentiation of murine hematopoietic progenitor cells. Experimental hematology. 2005 Feb;33(2):232–242. doi: 10.1016/j.exphem.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Forraz N, Pettengell R, McGuckin CP. Characterization of a lineage-negative stem-progenitor cell population optimized for ex vivo expansion and enriched for LTC-IC. Stem cells (Dayton, Ohio) 2004;22(1):100–108. doi: 10.1634/stemcells.22-1-100. [DOI] [PubMed] [Google Scholar]
- 29.Daldrup-Link HE, Rudelius M, Piontek G, Metz S, Brauer R, Debus G, et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology. 2005 Jan;234(1):197–205. doi: 10.1148/radiol.2341031236. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Boehmler AM, Seitz G, Kuci S, Wiesner T, Brinkmann V, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004 Jun 15;103(12):4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs CJ, Evans MJ, Daly BM. A hematopoietic stromal lesion associated with fractionated radiotherapy (FxRT): time- and dose-effects. Anticancer research. 2005 Jul-Aug;25(4):2801–2807. [PubMed] [Google Scholar]
- 32.Bastianutto C, Mian A, Symes J, Mocanu J, Alajez N, Sleep G, et al. Local radiotherapy induces homing of hematopoietic stem cells to the irradiated bone marrow. Cancer research. 2007 Nov 1;67(21):10112–10116. doi: 10.1158/0008-5472.CAN-07-2192. [DOI] [PubMed] [Google Scholar]
- 33.Singh AK, Savani BN, Albert PS, Barrett AJ. Efficacy of CD34+ stem cell dose in patients undergoing allogeneic peripheral blood stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant. 2007 Mar;13(3):339–344. doi: 10.1016/j.bbmt.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Kamel AM, El-Sharkawy N, Mahmoud HK, Khalaf MR, El Haddad A, Fahmy O, et al. Impact of CD34 subsets on engraftment kinetics in allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2005 Jan;35(2):129–136. doi: 10.1038/sj.bmt.1704755. [DOI] [PubMed] [Google Scholar]
- 35.Mohty M, Bilger K, Jourdan E, Kuentz M, Michallet M, Bourhis JH, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003 May;17(5):869–875. doi: 10.1038/sj.leu.2402909. [DOI] [PubMed] [Google Scholar]
- 36.Jin-Xiang F, Xiaofeng S, Jun-Chuan Q, Yan G, Xue-Guang Z. Homing efficiency and hematopoietic reconstitution of bone marrow-derived stroma cells expanded by recombinant human macrophage-colony stimulating factor in vitro. Experimental hematology. 2004 Dec;32(12):1204–1211. doi: 10.1016/j.exphem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Plett PA, Frankovitz SM, Orschell CM. Distribution of marrow repopulating cells between bone marrow and spleen early after transplantation. Blood. 2003 Sep 15;102(6):2285–2291. doi: 10.1182/blood-2002-12-3742. [DOI] [PubMed] [Google Scholar]
- 38.Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood. 2001 Oct 1;98(7):2108–2115. doi: 10.1182/blood.v98.7.2108. [DOI] [PubMed] [Google Scholar]
- 39.Dooner M, Cerny J, Colvin G, Demers D, Pimentel J, Greer D, et al. Homing and conversion of murine hematopoietic stem cells to lung. Blood cells, molecules & diseases. 2004 Jan-Feb;32(1):47–51. doi: 10.1016/j.bcmd.2003.09.014. [DOI] [PubMed] [Google Scholar]