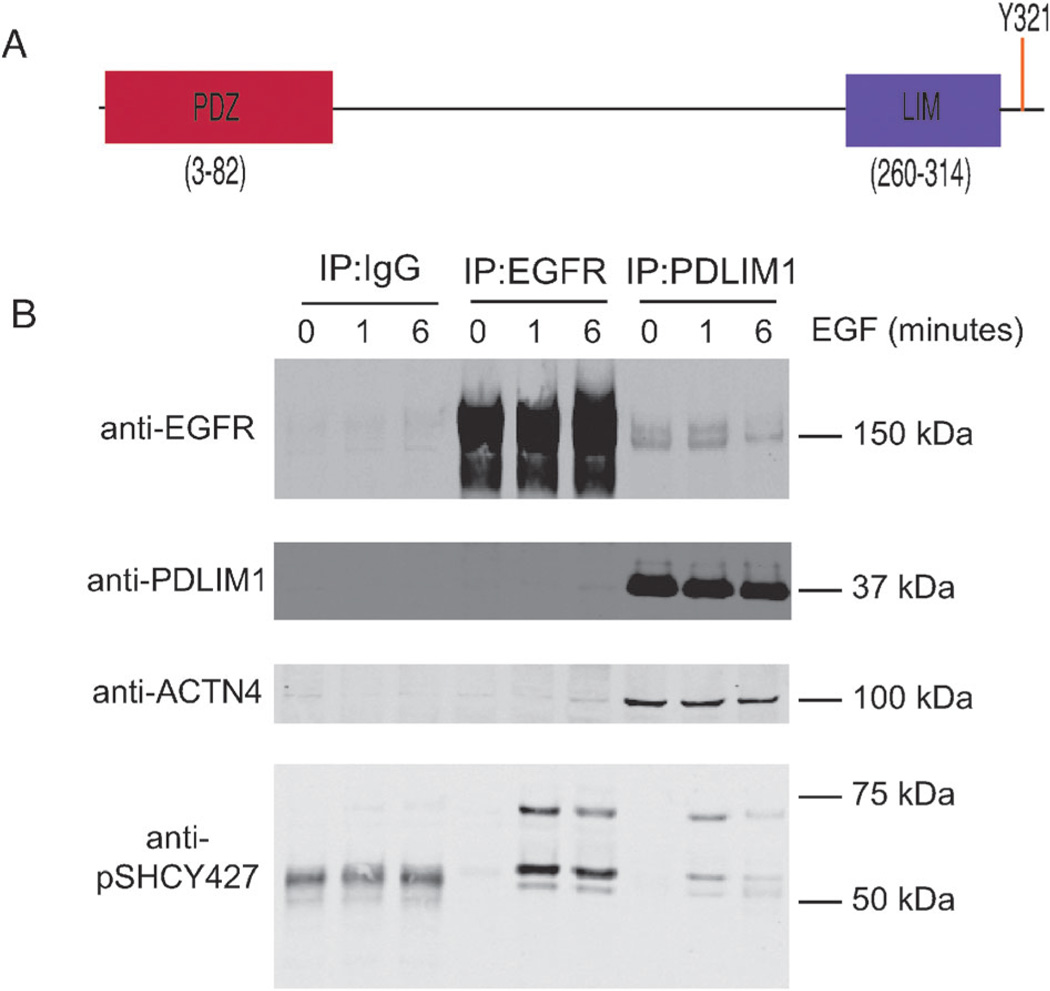
Fig. 4.
Co-immunoprecipitation of EGFR and PDLIM1. (A) The structure of PDLIM1, indicating the N-terminal PDZ domain and the C-terminal LIM domain. The phosphorylation site of interest, Y321, lays eight residues upstream of the C-terminus. (B) Confluent plates of HMECs were treated with either a media-control (0 minutes) or media supplemented with EGF (total concentration 100 ng mL−1) for 1 or 6 minutes, as indicated. Lysates were then immunoprecipitated with agarose beads conjugated to an IgG control, EGFR antibody, or PDLIM1 antibody, as indicated. Eluted proteins from the IP were than blotted for the presence of EGFR or PDLIM1, the known PDLIM1-interactor alpha-actinin 4, ACTN4, or the known EGFR-interactor SHC using a phospho-SHC Y427 antibody.