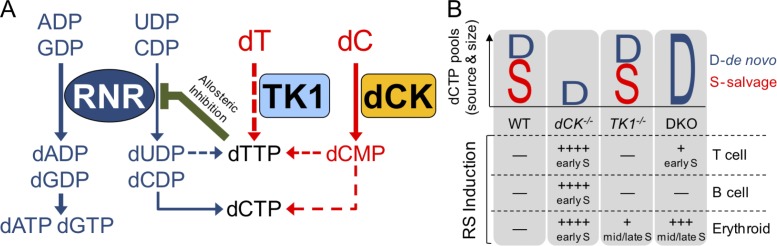
Figure 6.
Deoxyribonucleoside salvage kinases induce and resolve RS during hematopoiesis. (A) Under normal conditions, the RNR complex reduces purine ribonucleotide diphosphates (ADP and GDP) and pyrimidine ribonucleotide diphosphates (CDP and UDP) to contribute to dNTP pools (dATP, dGTP, dTTP, and dCTP). Although in hematopoietic cells RNR appears to be solely responsible for producing purine dNTP pools, the majority of dTTP pools are produced from salvaged thymidine, which is present in abundant amounts in thymus and BM. dCK may also contribute to dTTP pools as shown in Fig. 1 A and Fig. 2 C. Elevated dTTP levels prevent RNR from reducing CDP to dCDP and possibly UDP to dUDP via allosteric inhibition. To maintain dCTP pools, rapidly dividing hematopoietic cells rely on deoxycytidine salvage via dCK. (B) Graphical representation of the source (D-de novo, S-salvage) and size (height of D or S) of dCTP pools in various hematopoietic lineages. In the absence of dCK activity (dCK−/− column), dCTP pools become insufficient, leading to severe RS (++++) and DNA synthesis arrest in early S-phase in T cell, B cell, and erythroid cell precursors. In the absence of TK1 activity (TK1−/− column), dCTP pools are unaffected and only mild RS (+) occurs in late S-phase in erythroid precursors. The mild RS may be caused by an imbalanced dUTP/dTTP ratio in the absence of TK1. When both dCK and TK1 are inactivated (DKO column), de novo dCDP production is de-repressed and, subsequently, dCTP pools are restored to WT levels. DKO thymocytes have measurable, but overall mild, levels of RS (+) in early S-phase. The absence of NSP is well tolerated by B cell precursors, but it results in severe late S-phase RS (+++) in erythroid precursors.