Abstract
The serotonin receptor 2A gene polymorphism is associated with attentional processes in schizophrenia. However, the specificity of the underlying cognitive constructs affected within this domain requires further elucidation. We carried out the first investigation of whether the TC/CC genotype of the 5-HT2A T102C polymorphism confers impairments in early-onset schizophrenia (EOS; onset of psychotic symptoms before age 18) but not in healthy siblings, the putative mechanism being that serotonergic inhibitory modulation of prefrontal dopamine is impaired in the presence of the C allele which in turn is a genetic risk marker for schizophrenia. Fifty-three EOS outpatients and 46 of their non-psychotic siblings (no current Axis I diagnoses) were genotyped for 5-HT2A T102C polymorphism. The Positive and Negative Syndrome Scale (PANSS) was used to assess symptomatology severity. Diagnostic classification was based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Structured Clinical Interview. The Degraded-Stimulus Continuous Performance Test (DS-CPT) was used to measure sustained focused attention. As predicted, EOS probands produced fewer correct responses (hit rate) and demonstrated poorer perceptual sensitivity compared with the healthy siblings. The C allele at codon 102 was associated with fewer correct responses compared with the TT genotype. There was no significant relationship between the polymorphism and clinical parameters, as measured using the PANSS. Our findings suggest that the C allele may be related to sustained attentional impairments in EOS.
Keywords: adolescence, attention, biological relatives, gene polymorphism, T102C; HTR2A, Schizophrenia
A role for serotonin receptors in schizophrenia has long been suggested by the fact that some antipsychotic medications act upon serotonin receptors, and some serotonin-like molecules (e.g. D-lysergic acid diethylamide (LSD)) have prominent psychotogenetic effects [1–3]. One polymorphism that has been intensely studied is a single nucleotide polymorphism (SNP) at codon 102 (rs6313; either thymine (T) or cytosine (C)) on the serotonin receptor type 2A (HTR2A) gene located at 13q14–q21 [4]. The gene encoding HTR2A contains a polymorphism that alters HTR2A functioning and may therefore be a functional candidate gene. There is some evidence linking this variant to psychiatric diagnoses, outcomes, and related personality phenotypes [5, 6]. A meta-analysis implicated an association between the C allele of the T102C polymorphism and schizophrenia [7]. Because serotonin modulates dopaminergic neurons [8], it is plausible that the polymorphism of the serotonin receptor gene may be associated with clinical features and specific cognitive functions in individuals with schizophrenia. This is also evident from animal studies which suggest that dopamine and 5-HT play an important role in frontal lobe cortical processing, particularly in speed of processing, attention, and working memory [9]. Golimbet and colleagues [10] showed an association of heterozygotes A1/A2 (T102C) with personality traits (measured using the multiphasic personality assessments and Eysenck Personality Inventory) in healthy individuals. The C allele at codon 102 has been associated with poor prognosis [11], and earlier contact with schizophrenia services [10]. However, one study has reported an association of the T allele with higher frequency of hospitalization [12]. In contrast, two studies reported no association of 5-HT2A with general psychopathology (as measured using the Positive and Negative Syndrome Scale; PANSS) [13] or suicidality [14].
Studies have investigated the impact of HTR2A polymorphisms specifically on executive function, memory, and attention function in schizophrenia. Üçok and colleagues [15] reported that the TC genotype was associated with poorer performance on sustained attention (continuous performance test) and abstraction (Wisconsin card sorting test; WCST) compared with both homozygous groups, TT and CC, in 82 outpatients with schizophrenia. Similarly, Lane and co-workers [16] found that TC individuals produced 25% more perseverative errors than the TT genotype. A recent study comparing short-term verbal memory in schizophrenia spectrum disorder and controls reported an association of the CC genotype with worse performance in overall productivity and immediate reproduction of 10 unconnected nouns, in comparison with the superior-performing heterozygous group [17]. The T102C CC genotype is reported at higher frequency in schizophrenia, and is therefore the subject of intense interest [18, 19]. However, the specific role of HTR2A on measures relating to attentional processes is not clearly defined and requires further elucidation.
Two suggested strategies for improving the ability to link risk genes to psychosis have been to study early-onset groups and to consider cognitive endophenotypes rather than the clinical diagnosis. Investigating selective deficits in cognitive function in early-onset schizophrenia (EOS; onset of psychotic symptoms before the age of 18 years) is particularly informative, as the early stages of schizophrenia run alongside a critical period of continued brain maturation [20] associated with increased cognitive abilities in abstract reasoning, attention, working memory, and executive function [21]. EOS is also associated with higher genetic loading, neurodevelopmental deviance, severe general psychopathology, and poor psychosocial outcome [22–24]. Further, EOS provides a unique opportunity to study disease correlates with less risk of secondary influences from disease-associated alterations of environment [24, 25]. EOS is associated with generalized cognitive impairments across a broad array of ability domains [26, 27]. In particular, the continuous performance test (CPT) may index genetic risk for schizophrenia [28–30]. The CPT is a measure of sustained focused attention and impulsivity, and is considered a neurocognitive endophenotype for genetic susceptibility to schizophrenia [31–33]. Cognitive deficits in sustained attention using ungraded or degraded CPT have also been identified in clinically unaffected relatives of schizophrenia probands, suggesting a heritable risk of the illness [34–42].
Since genetic variation in 5-HT2A functioning has the capacity to impact CPT, to test if any associations observed in probands reflect risk for schizophrenia or are a function of active disease, we also examined gene-cognition relationships in non-psychotic siblings of EOS patients. Our central question was whether the TC/CC genotype of the 5-HT2A T102C polymorphism confers impairments in EOS but not in healthy siblings, the putative mechanism being that serotonergic modulation of prefrontal dopamine is impaired in the presence of the C allele which in turn is a genetic risk marker for schizophrenia. Thus we hypothesized that performance on the degraded stimulus continuous performance test (DS-CPT), which relies on ‘bottom up’ processing and reduces working memory demands, would be impaired in EOS and, to a lesser degree, in their healthy first-degree relatives. The association of sustained visual attention with genetic risk is more etiologically complex; we predicted that sustained attention deficits would be associated with genetic risk alleles for schizophrenia. Although the CC genotype has shown to be associated with clinical features of the syndrome, poor cognitive performance in schizophrenia has been identified in individuals with the TC and CC genotype. Therefore, we predicted that individuals with the C allele will perform worse than those with the TT genotype. We also explored the association of 5-HT2A genotype on clinical parameters in this enriched population.
MATERIALS & METHODS
Participants
Fifty-three patients (29 boys, 24 girls) with early onset schizophrenia (psychosis first presenting before age 18), and diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders [43] were recruited through clinicians’ referrals in adolescent services in South London (39% Caucasian, 43% Black/African Caribbean; 14% Asian, 4% mixed; mean (± standard deviation) age 17.25±1.31 years; age range 14.1–21.2 years; mean illness duration 23.5±18.5 months; average number of hospitalizations (1.2±0.96). Forty-six (n=46) non-psychotic siblings of EOS probands (mean age 19.4±3.37 years) also participated in the Vulnerability Indicators in Psychosis Study (VIPS). This study examines clinical, cognitive and brain structural and functional characteristics of genetic liability and disease expression in EOS using a family-based design. The study protocol was approved by the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee. Written informed consent or assent was obtained from all participants in the study in accordance with the Declaration of Helsinki (http://www.wma.net). The exclusion criteria for EOS patients and biological relatives included: (a) a history of neurological disorders or head injury resulting in a loss of consciousness for more than 1 hour; (b) family history of hereditary neurological disorders; (c) full-scale IQ less than 70, and (d) DSM-IV criteria for psychoactive substance abuse in the preceding 6 months or the presence of co-morbid Axis I disorders.
Clinical Evaluation
A trained child and adolescent psychiatrist administered the structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) DSM-IV [43] to verify schizophrenia diagnosis and to screen for inclusion criteria. At the time of assessment, fifty EOS probands were on atypical medication, one proband was on a typical medication and two were unmedicated. Forty-six healthy siblings (no current Axis I diagnosis), assessed by personal interview using the Structured Clinical Interview for DSM-IV Axis I Disorders (non-patient research version) [44], were included in this study. The healthy siblings were not taking any psychotrophic medications at the time of entry to the study. Information about age of onset, number and type of previous episodes, number of hospital admissions and current medication were collected from medical notes. The current antipsychotic dose was calculated according to the Comparable Daily Dose [45]. Clinical symptoms were assessed using the PANSS [46], comprising of positive, negative and general psychopathology subscales, and were relatively stable in all patients for a minimum period of 6 months. All participants were assessed using the Global Assessment of Functioning (GAF) [47]. The GAF score (mean±SD) for EOS patients and siblings was 56.13±13.39 and 82.65±6.21, respectively. Table 1 shows the demographic characteristics of the sample according to genotype.
Table 1.
Demographic and cognitive characteristics by genotype for EOS and healthy siblings.
| EOS probands | Healthy Siblings | P statistic | |||
|---|---|---|---|---|---|
| TT | TC/CC | TT | TC/CC | ||
| Gender (M/F) | 3/7 | 24/19 | 8/2 | 20/16 | 0.07 |
| Age (years) | 17.26+1.23 | 17.24+1.39 | 20.77+3.43 | 18.18+3.32 | 0.09 |
| Education (years) | 10.2±1.1 | 10.5±1.0 | 10.2±1.13 | 10.56±l.04 | 0.35 |
| Full-Scale IQ | 91+17.12 | 99.07+24.29 | 92+19.39 | 93.02+16.85 | 0.324 |
| *Positive | 9.1±1.96 | 9.31+2.12 | - | - | 0.536 |
| *Negative | 14.6±4.88 | 14.78+5.57 | - | - | 0.521 |
| *General psychopathology | 22.6+4.55 | 24.92+5.18 | - | - | 0.643 |
| Proportion of atypical antipsychotics | 6 | 23 | - | - | 0.43 |
| CPT-hit rate | 20.4+0.72 | 17.87+3.52 | 19.5+0.84 | 19.4+0.86 | 0.024 |
| CPT-Sensitivity | 2.05+1.86 | 2.49+2.1 | 3.28+2.48 | 3.85+1.48 | 0.349 |
| CPT-Response criterion | 0.97+0.07 | 0.97+0.09 | 0.99+0.01 | 0.97+0.09 | 0.761 |
PANSS, Positive and Negative Syndrome Scale; NA, not applicable.
Neuropsychological Measures
All participants underwent cognitive assessment by the same qualified psychologist. Prior to neurocognitive assessment, patients with a diagnosis of schizophrenia were evaluated weekly over a period of one month to ensure that they were in partial remission of the illness and that there had been no change to their medication type and dose where applicable.
General intellectual ability was measured using age-appropriate forms of the Wechsler Intelligence Scales to derive full-scale IQ [48, 49]. Visual sustained attention was assessed using the Degraded-Stimulus version of the Continuous Performance Test [50–52]. In the DS-CPT, subjects were shown a series of single numerical digits with degraded resolution. The stimuli were presented for 40 ms each at a rate of 1 per second on a computer screen situated 1 meter away from the seated participants. The participant was instructed to respond as quickly as possible by pressing a button when a designated target numeral “0” appeared. The target numeral occurred in a quasi-random fashion in 25% of the 480 trials. To allow the participant to become familiar with the general set-up of the task, all participants underwent two practice trials followed by four test trials. The analysis was conducted on average number of correct responses (“hit rate”) across four trials, perceptual sensitivity (d-prime) (referring to an individual’s ability to discriminate target stimuli from non-targets stimuli) and response criterion (or response threshold reflecting overall tendency to respond and the amount of perceptual evidence a person requires to decide that a stimulus is a target). The DS-CPT is considered a sensitive assessment of subtle deficits in sustained visual attention with minimal short-term memory and working memory demands [50, 51].
Genotyping
DNA was extracted from cheek swabs from 99 participants using standard methods [52]. Genotypying of the single nucleotide polymorphism for rs6313 was performed by a TaqMan SNP genotyping assay (assay ID: C-3042197-1) (Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA). 10 ng DNA was used in a 10-μl reaction, according to the manufacturer’s instructions. End-point analysis was performed on an ABI 7900 HT PCR system and a probability >95% was attained for the sequence detection system package. Genotype frequencies for rs6313 were 0.21 (TT), and 0.79 (TC/CC). The genotype frequencies were comparable with Hap-Map population genome build 132 dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=6313). The genotype distribution of rs3613 SNP in 5-HT2A was in Hardy-Weinberger equilibrium (P = 0.70). Genotype frequencies were comparable to those reported in other studies [15].
Statistical Analyses
Statistical analyses were conducted using Statistical Packages for Social Sciences version 18 (SPSS, Chicago, IL, USA). To study the effect of genotype and type of diagnosis on our outcome measures we used a generalized estimating equation model with an exchangeable within-subject working correlation matrix and robust standard errors [53,54] to account for familial inter-correlation between the EOS probands and their siblings. The Hubert White sandwich estimator was used which provides standard errors that are robust to possible misspecification of the correlation matrix. Unlike repeated measurement ANOVA, a generalized estimating equation allows for several observations per case and uses a full case analysis [54]. We used a factorial design to study group (EOS vs. healthy siblings) by genotype (TT, TC/CC) interaction on DS-CPT measures. On the basis of significant main effects or interactions, Bonferroni post-hoc comparisons were performed (P-values were multiplied by the number of pairwise comparisons). The magnitude of pairwise differences between EOS and siblings was assessed using measures of effect size based on Cohen’s d [55].
RESULTS
Sample characteristics
Table 1 shows the clinical and demographic characteristics by genotype for the sample. There was no significant difference between EOS probands and siblings with age (P=0.389, education (P=0.35), IQ (P=0.32). There were no differences in allelic distribution between patients and siblings (P=0.59). Genotype did not vary with sex (Wald χ2=.81, df=1, P=0.36), socio-economic status (Wald χ2=.12, df=1, P=0.73), ethnicity (Wald χ2=3.71, df=1, P=0.07), or full-scale IQ (Wald χ2=0.71, df=1, P=0.39). There was no association of HTR2A with PANSS positive score, negative score, or general psychopathology score (all P>0.05). There was no significant association between medication and performance on the DS-CPT (Pearson’s r=0.21, P=0.43).
Sustained attention
EOS probands scored significantly worse on the DS-CPT compared with the sibling group (Wald χ2=5.84, df=1, P=0.016; Cohen’s d=0.51). There was a main effect of group on perceptual sensitivity (Wald χ2=5.71, df=1, P=0.01, Cohen’s d=0.69), but not response criterion (Wald χ2=0.21, df=1, P=0.64), where healthy siblings were able to detect targets and reject distracters more efficiently compared to EOS probands (Figure 1). There was no significant main effect for CPT sensitivity (Wald χ2=0.87, df=1, P=0.34) or response criterion (Wald χ2=0.09, df=1, P=0.76).
Figure 1.
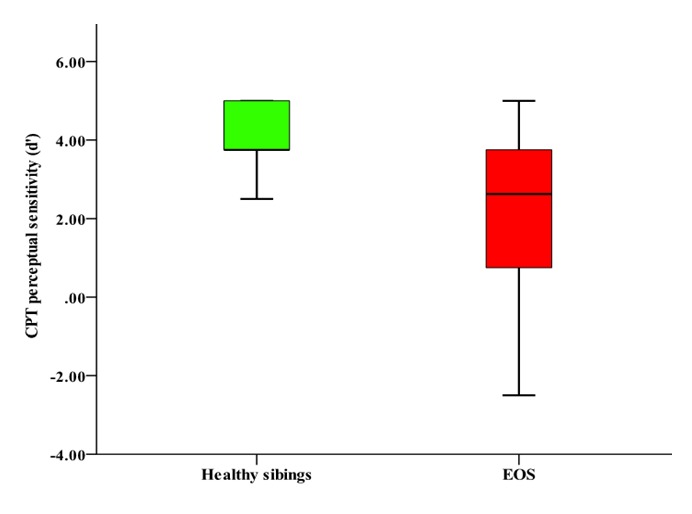
DS-CPT Perceptual Sensitivity (d’) Performance in Patients with EOS and their Healthy Siblings.
There was a main effect of 5-HT2A genotype on DSCPT (Wald χ2=5.12, df=1, P=0.024), with individuals with TT produced a greater number of correct “hits” than TC (TT vs. TC; Cohen’s d=0.28) and CC (TT vs. CC; Cohen’s d=0.48) genotype. Analysis of DS-CPT hit rate showed a significant interaction between genotype and group (Wald χ2=4.91, df=1, P=0.027). Post-hoc analysis showed that healthy siblings with TT genotype outperformed EOS probands with TC/CC genotype (P=0.05; Cohen’s d=0.63). Healthy siblings with TC/CC genotype outperformed EOS in the same genotype (P=0.039; Cohen’s d=0.65). Figure 2 shows genotype by group interaction on DS-CPT hit rate.
Figure 2.
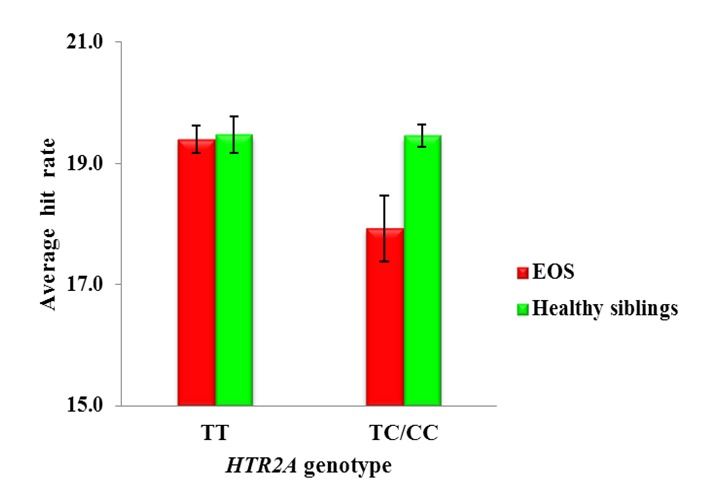
DS-CPT Hit Rate and HTR2A Gene Polymorphism in EOS and Healthy Siblings.
DISCUSSION
This study aimed to assess whether the TC/CC genotype of the 5-HT2A serotonin receptor gene confers impairments in EOS but not in healthy siblings. Our study was unique since we investigated the association of the T102C polymorphism of the 5-HT2A receptor gene on visual sustained in patients with early onset schizophrenia and their non-psychotic siblings. We reported on several findings. First, we found an association of the C allele of the 5-HT2A polymorphism with poor performance on CPT hit rate in EOS probands. Second, we found that EOS with TC/CC genotype produced fewer correct responses and were less efficient in detecting targets and rejecting distracters than healthy siblings with the same genotype.
Consistent with the findings by Üçok and colleagues [15], we found a relationship between the C allele and visual sustained attention. In previous studies, the C allele has shown to be related with the clinical expression of schizophrenia [10, 11]. Our study suggests that the same allele may also be associated with worse performance on sustained focused attention. An earlier study reported poor performance in CC homozygotes in a short-term memory productivity in schizophrenia and healthy controls [17]. The allocation of attentional resources is required when undergoing any neurocognitive task. Indeed, animal model of disease, point out that serotonin receptor 2A receptors plays an important role in the modulation of attentional processes [56].
In line with previous findings [15], we did not detect any association between genotype distribution and clinical features, as measured using the PANSS scores. The findings may reflect the antipsychotic treatment rather than genotype differences.
We utilized a cognitive neuroendophenotype which measures sustained focused attention [32,33]. Our study reported a graded differential role of HTR2A on sustained attention, whereby individuals with TT genotype showed superior performance on the number of correct hits compared to individuals with the TC/CC genotype. Üçok and colleagues [15] reported that individuals with TT and TC genotype showed a lower hit rate on the successive discrimination vigilance task (CPT-ZA) compared with CC genotype in schizophrenia patients. They also reported that the TC genotype showed more commission errors compared with TT and CC genotype [15]. However, earlier clinical and cognitive studies have reported that the TC and CC genotype is associated with poor performance compared with their counterparts [10, 11, 17, 57]. A possible explanation for discrepancies in findings may be owing to the multivariate nature and task requirements of the CPT, some of which are more sensitive to attention disturbances, while others constitute several cognitive constructs. Notably, although the CPT-ZA is considered a measure of sustained attention, it involves components of short-term memory and working memory, which may place greater information processing loading on subjects. Indeed, the different versions of the CPT test evaluate different components of sustained attention, where the inclusion of a working memory component in the case of CPT-AX [37,51, 58] or CPT-IP version [59], result in performance remaining stable between psychotic states and periods of remission. The DS-CPT used in our study relies on ‘bottom up’ processing and places minimal working memory demands [60–62]. Therefore, the CPTZA task may provide meaningful insights into executive functional processes, which involve memory, but may not be a pure measure of sustained attention. Our finding provides support for an association of HTR2A with sustained focused attention. This may suggest a specific influence of HTR2A in discriminating predesigned targets (DS-CPT).
There are a number of study limitations that merit comment. First, our sample size was relatively small for a genetic study. Of note, we studied a rare, severe and chronic form of schizophrenia which occurs in only 5% of all schizophrenia cases [63–66]. Yet, there is a need to replicate this study with a larger sample. We recruited subjects by carefully reviewing clinical documentation prior to conducting neuropsychological assessment on EOS and unaffected siblings to account for confounding factors and removed the effect of ascertainment bias. Second, our EOS patients were taking antipsychotic medication which may have influenced the results. However, we failed to detect an association of the HTR2A gene polymorphism on PANSS scores in EOS. Third, there were very few individuals in either group with the TT genotype, indicating that there was limited statistical power in detecting differences between TT and TC/CC genotype; therefore there is a pressing need to conduct larger prospective studies to validate our findings. In spite of these limitations, this study suggests that the HTR2A is associated with visual sustained/focused attention in patients with schizophrenia.
In conclusion, our study suggests that there is an association of the C allele with poor performance of individuals on visual sustained attention. There was no influence of medication or psychopathology scores on cognitive performance. A larger study with additional measures of visual sustained attention with a larger sample is needed to confirm and further validate our findings. Future studies should continue to pursue more genes involved in the modulation of attentional measures that may confer a greater risk in schizophrenia.
Acknowledgments
We would like to thank all families who contributed to the VIPS study. This work was supported by a departmental fund at King’s College London, Institute of Psychiatry, London, UK. We are very grateful to Professors Sophia Frangou and David A Collier for their research supervision, and useful comments on earlier drafts of this manuscript. We thank Dr Armin Raznahan (Child Psychiatry Branch, National Institutes of Health) for his useful comments on our manuscript. We would also like to thank Dr Lisa Burke for conducting the clinical interviews in this study.
NSV is supported by the Fulbright Distinguished Scholar Award by the US-UK Fulbright Commission. AAC thanks the Motor Neurone Disease of Great Britain and Northern Ireland and the ALS Association for support. We thank the NIHR specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry, King’s College London. The research leading to results (ALS) has received funding from the European Community’s Health Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 259867.
Footnotes
Conflict of interest statement
No conflicts declared.
References
- [1].Meltzer HY, Nash J. The effect of antipsychotic drugs on serotoninergic receptors. Pharmacol Rev. 1991;43:587–604. [PubMed] [Google Scholar]
- [2].Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, Kraus JE. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998;44:1099–1117. doi: 10.1016/s0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- [3].Harrison PJ. Neurochemical alterations in schizophrenia affecting the putative receptor targets of atypical antipsychotics. Focus on dopamine (D1, D3, D4) and 5-HT2a receptors. Br J Psychiatry (Suppl) 1999;1:12–22. [PubMed] [Google Scholar]
- [4].Hsieh CL, Bowcock AM, Farrer LA, Hebert JM, Huang KN, Cavalli-Sforza LL, Julius D, Francke U. The serotonin receptor subtype 2 locus HTR2 is on human chromosome 13 near genes for esterase D and retinoblastoma-1 and on mouse chromosome 14. Soma Cell Mol Genet. 1990;16:567–574. doi: 10.1007/BF01233097. [DOI] [PubMed] [Google Scholar]
- [5].Patel NH, Vyas NS, Nijran KS, Puri BK, Al-Nahhas A. PET Imaging in Schizophrenia: A New Perspective. J Nucl Med. 2010;51(4):511–520. doi: 10.2967/jnumed.109.066076. [DOI] [PubMed] [Google Scholar]
- [6].Vyas NS, Patel NH, Nijran KS, Al-Nahhas A, Puri BK. The Use of Positron Emission Tomography Imaging in Understanding Cognition, Genetics, and Psychopharmacology Interventions in Schizophrenia. Expert Rev Neurother. 2011;11(1):37–51. doi: 10.1586/ern.10.160. [DOI] [PubMed] [Google Scholar]
- [7].Abdolmaleky HM, Faraone SV, Glatt SJ, Tsuang MT. Meta analysis of association between the T102C polymorphism of the 5-HT2A receptor gene and schizophrenia. Schizophr Res. 2004;67:53–62. doi: 10.1016/s0920-9964(03)00183-x. [DOI] [PubMed] [Google Scholar]
- [8].Nocjar C, Roth BL, Pehek EA. Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- [9].Rutschmann J, Cornblatt B, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia: findings with two visual continuous performance tests in a new sample. J Abnorm Child Psychol. 1986;14:365–385. doi: 10.1007/BF00915432. [DOI] [PubMed] [Google Scholar]
- [10].Golimbet VE, Alfimova MV, Mitiushina NG. Polymorphism of the serotonin 2A receptor gene (5HTR2A) and personality traits. Mol Biol (Mosk) 2004;38:404–412. [PubMed] [Google Scholar]
- [11].Joober R, Benkelfat C, Brisebois K, Toulouse A, Turecki G, Lal S, Bloom D, Labelle A, Lalonde P, Fortin D, Alda M, Palmour R, Rouleau GA. T102C polymorphism in the 5-HT2A gene and schizophrenia: relation to phenotype and drug variability. J Psychiatry Neurosci. 1999;24:141–146. [PMC free article] [PubMed] [Google Scholar]
- [12].Herken H, Erdal ME, Erdal N, Aynacioglu S. T102C polymorphisms at the 5-HT2A receptor gene in Turkish schizophrenia patients: A possible association with prognosis. Neuropsychobiology. 2003;4:27–30. doi: 10.1159/000068872. [DOI] [PubMed] [Google Scholar]
- [13].Chen RY, Sham P, Chen EY, Li T, Cheung EF, Hui TC, Kwok CL, Lieh-Mak F, Zhao JH, Collier D, Murray R. No association between T102C polymorphism of serotonin-2A receptor gene and clinical phenotypes of Chinese schizophrenic patients. Psychiatry Res. 2001;105:175–185. doi: 10.1016/s0165-1781(01)00343-2. [DOI] [PubMed] [Google Scholar]
- [14].Ertugrul A, Kennedy JL, Masellis M, Basile VS, Jayathilake K, Meltzer HY. No association of the T102C polymorphism of the serotonin 2A receptor gene (HTR2A) with suicidality in schizophrenia. Schizophr Res. 2004;69:301–305. doi: 10.1016/s0920-9964(03)00126-9. [DOI] [PubMed] [Google Scholar]
- [15].Üçok A, Alpsan H, Cakir S, Saruhan-Direskeneli G. Association of a serotonin receptor 2A gene polymorphism with cognitive functions in patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144:704–707. doi: 10.1002/ajmg.b.30463. [DOI] [PubMed] [Google Scholar]
- [16].Lane HY, Liu YC, Huang CL, Chang YL, Chang L, Chang L, Chang YC, Chang WH. Prefrontal executive function and D(1), D(3), 5-HT(2A) and 5-HT(6) receptor gene variations in healthy adults. J Psychiatry Neurosci. 2008;33:47–53. [PMC free article] [PubMed] [Google Scholar]
- [17].Alfimova MV, Monakhov MV, Abramova LI, Golubev SA, Golimbet VE. Polymorphism of serotonin receptor genes (5-HTR2A) and dysbindin (DTNBP1) and individual components of short-term verbal memory processes in schizophenia. Neurosci Behav Physiol. 2010;40:934–940. doi: 10.1007/s11055-010-9348-7. [DOI] [PubMed] [Google Scholar]
- [18].Inayama Y, Yoneda H, Sakai T, Ishida T, Nonomura Y, Kono Y, Takahata R, Koh J, Sakai J, Takai A, Inada Y, Asaba H. Positive association between a DNA sequence variant in the serotonin 2A receptor gene and schizophrenia. Am J Med Genet. 1996;67:103–105. doi: 10.1002/(SICI)1096-8628(19960216)67:1<103::AID-AJMG18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [19].Williams J, Spurlock G, McGuffin P, Nothen M, Owen MJ. Meta-analysis of association between the 5-HT2A receptor T102C polymorphism and schizophrenia. European Multicentre Association Study of Schizophrenia (EMASS Group) Lancet. 1997;26:1221. doi: 10.1016/s0140-6736(05)62413-0. [DOI] [PubMed] [Google Scholar]
- [20].Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- [21].Vyas NS, Patel NH, Puri BK. Neurobiology and phenotypic expression in early onset schizophrenia. Early Interv Psychiatry. 2011;5:3–14. doi: 10.1111/j.1751-7893.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- [22].Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- [23].Vyas NS, Hadjulis M, Vourdas A, Byrne P, Frangou S. The Maudsley early onset schizophrenia study. Predictors of psychosocial outcome at 4-year follow-up. Eur Child Adolesc Psychiatry. 2007;16:465–470. doi: 10.1007/s00787-007-0621-4. [DOI] [PubMed] [Google Scholar]
- [24].Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives froom structural neuroimaging studies. Schizophr Bull. 2011;37:504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kenny JT, Friedman L, Findling RL, Swales TP, Strauss ME, Jesberger JA, Schulz SC. Cognitive impairment in adolescents with schizophrenia. Am J Psychiatry. 1997;154:1613–1615. doi: 10.1176/ajp.154.11.1613. [DOI] [PubMed] [Google Scholar]
- [26].Rapoport JL, Giedd JN, Gogtay N. The neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012 2012 Apr 10; doi: 10.1038/mp.2012.23. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumra S, Wiggs E, Bedwell J, Smith AK, Arling E, Albus K, Hamburger SD, McKenna K, Jacobsen LK, Rapoport JL, Asarnow RF. Neuropsychological deficits in pediatric patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified. Schizophr Res. 2000;42:135–144. doi: 10.1016/s0920-9964(99)00118-8. [DOI] [PubMed] [Google Scholar]
- [28].Brickman AM, Buchsbaum MS, Bloom R, Bokhoven P, Paul-Odouard R, Haznedar MM, Dahlman KL, Hazlett EA, Aronowitz J, Heath D, Shihabuddin L. Neuropsychological functioning in first-break, never-medicated adolescents with psychosis. J Nerv Ment Dis. 2004;192:615–622. doi: 10.1097/01.nmd.0000138229.29157.3e. [DOI] [PubMed] [Google Scholar]
- [29].Rhinewine JP, Lencz T, Thaden EP, Cervellione KL, Burdick KE, Henderson I, Bhaskar S, Keehlisen L, Kane J, Kohn N, Fisch GS, Bilder RM, Kumra S. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. 2005;58:705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- [30].Karatekin C, White T, Bingham C. Divided attention in youth- onset psychosis and attention deficit/hyperactivity disorder. J Abnorm Psychol. 2008;117:881–895. doi: 10.1037/a0013446. [DOI] [PubMed] [Google Scholar]
- [31].Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Horan WP, Greenwood TA, Braff DL, Gur RE, Green MF. The use of neurocognitive endophenotypes in large-scale family genetic studies of schizophrenia. In: Ritsne MS, editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. 2009. pp. 177–193. [Google Scholar]
- [34].Rutschmann J, Cornblatt BA, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Arch Gen Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- [35].Mirsky AF, Yardley SL, Jones BP, Walsh D, Kendler KS. Analysis of the attention deficit in schizophrenia: A study of patients and their relatives in Ireland. J Psychiat Res. 1995;29:23–42. doi: 10.1016/0022-3956(94)00041-o. [DOI] [PubMed] [Google Scholar]
- [36].Keefe RS, Silverman JM, Mohs RC, Siever LJ, Harvey PD, Friedman L, Lees Roitman SE, DuPre RL, Smith CJ, Schmeidler J, David KL. Eye tracking, attention, and schizotypal symptoms in nonpsychotic relatives of patients with schizophrenia. Arch Gen Psychiatry. 1997;54:169–176. doi: 10.1001/archpsyc.1997.01830140081014. [DOI] [PubMed] [Google Scholar]
- [37].Finkelstein JR, Cannon TD, Gur RE, Gur RC, Moberg P. Attentional dysfunctions in neuroleptic-naïve and neuroleptic-withdrawn schizophrenic patients and their siblings. J Abnorm Psychol. 1997;106:203–212. doi: 10.1037//0021-843x.106.2.203. [DOI] [PubMed] [Google Scholar]
- [38].Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998;155:1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- [39].Franke P, Maier W, Hardt J, Hain C, Cornblatt BA. Attentional abilities and measures of schizotypy: their variation and covariation in schizophrenic patients, their siblings, and normal control subjects. Psychiatry Res. 1994;54:259–272. doi: 10.1016/0165-1781(94)90020-5. [DOI] [PubMed] [Google Scholar]
- [40].Laurent A, Saoud M, Bougerol T, d’Amato T, Anchisi A, Biloa-Tang M, Dalery J, Rochet T. Attentional deficits in patients with schizophrenia and in their non-psychotic first-degree relatives. Psychiatry Res. 1999;89:147–159. doi: 10.1016/s0165-1781(99)00109-2. [DOI] [PubMed] [Google Scholar]
- [41].Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first- degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- [43].First M, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York, NY: New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders - Research Version, Patient Edition (SCIDI/P) [Google Scholar]
- [44].First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV –TR Axis 1 Disorders – Research Version, Non-patient Edition (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- [45].Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 10th rev ed. Toronto: Hogrefe and Huber; 2000. [Google Scholar]
- [46].Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- [47].American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- [48].Wechsler D. Wechsler Adult Intelligence Scale-Revised. Ohio: The Psychological Corporation Cleveland; 1991. [Google Scholar]
- [49].Wechsler D. Manual for the Wechsler Intelligence Scale for Children - Third Edition (WISCIII) UK Edition. Sidcup, Kent: The Psychological Corporation; 1992. [Google Scholar]
- [50].Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrements over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- [51].Nuechterlein KH. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Neuropsychology, Psychophysiology and Information Processing Handbook of Schizophrenia. Vol. 5. Amsterdam: Elsevier; 1991. pp. 397–433. [Google Scholar]
- [52].Nuechterlein KH, Asarnow RF. Manual and Computer Program for the UCLA Continuous Performance Test. 1992. UCLA, Los Angeles, Unpublished manual and program. [Google Scholar]
- [53].Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior Genetics. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- [54].Nelder J, Wedderburn R. Generalized linear models. J R Stat Soc Ser A. 1972;135:370–384. [Google Scholar]
- [55].Hardin J, Hilbe J. Generalized Estimating Equations. London: Chapman and Hall; 2003. [Google Scholar]
- [56].Cohen J. Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- [57].Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- [58].Alfimova MV, Golimbet VE, Mitiushina NG. Polymorphism of the serotonin receptor (5-HTR2A) gene and verbal fluency in normalcy and schizophrenia. Mol Biol (Mosk) 2003;37:68–73. [PubMed] [Google Scholar]
- [59].Epstein JI, Keefe RSE, Roitman SL, Harvey PD, Mohs RC. Impact of neuroleptic medications on continuous performance test measures in schizophrenia. Biol Psychiatry. 1996;39:902–905. doi: 10.1016/0006-3223(95)00588-9. [DOI] [PubMed] [Google Scholar]
- [60].Cornblatt B, Obuchowski M, Schnur B, O’Brien JD. Attention and clinical symptoms in schizophrenia. Psychiatr Quart. 1997;68:343–359. doi: 10.1023/a:1025495030997. [DOI] [PubMed] [Google Scholar]
- [61].Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci USA. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttenen T, Rabe-Hesketh S, Murray RM. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999;56:457–463. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- [64].Vyas NS, Kumra S, Puri BK. What insights can we gain from studying early-onset schizophrenia? The neurodevelopmental pathway and beyond. Expert Rev Neurother. 2010;10:1243–1247. doi: 10.1586/ern.10.109. [DOI] [PubMed] [Google Scholar]
- [65].Vyas NS, Shamsi SA, Malhotra AK, Aitchison KJ, Kumari V. Can genetics inform the management of cognitive deficits in schizophrenia? J Psychopharmacol. 2012;26:334–348. doi: 10.1177/0269881111434623. [DOI] [PubMed] [Google Scholar]
- [66].Childs B, Scriver CR. Age at onset and causes of disease. Perspect Biol. 1986 doi: 10.1353/pbm.1986.0056. [DOI] [PubMed] [Google Scholar]


