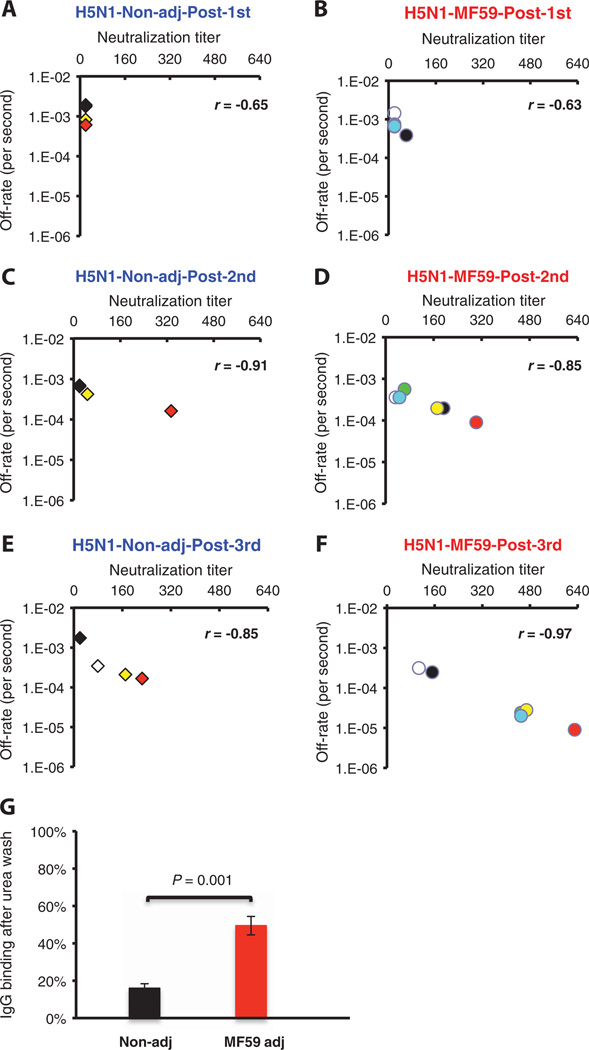
Fig. 7.
Kinetics of antibody affinity maturation to HA after multiple immunizations with an MF59-adjuvanted and unadjuvanted H5N1 subunit vaccine and its correlation with in vitro VN. (A to F) Sequential SPR analysis of vaccine sera (after the first, second, and third vaccination with unadjuvanted or MF59-adjuvanted H5N1 vaccine) was performed with properly folded H5N1 HA1 (A/Vietnam/1203/2204) (13, 47). Tenfold-diluted individual sera from three arms of the NVD vaccine trial at 28 days after each immunization were evaluated. After binding of the sera to the immobilized ligand, antibody off-rate constants were calculated with a heterogeneous sample model as described in Materials and Methods. Values on the x axis denote the end-point VN titers (mean of three replicates) with individual sera in a VN assay performed with A/Vietnam/1203/2004-rgH5N1×PR8 reassorted virus. Data plotted are shown for four individuals from the unadjuvanted arm (A, C, and E) and six individuals from the MF59- adjuvanted H5N1 vaccine arm (B, D, and F) in the NVD trial after first, second, and third immunizations. To follow the serum VN titer and corresponding polyclonal serum off-rate constants after every vaccination, the values for each individual vaccinee are depicted with the same colored symbol after the first, second, and third immunization in the three corresponding figures of each arm. Statistical analysis of the off-rate constants between MF59-adjuvanted and unadjuvanted vaccine groups after each vaccination showed statistical significance betweenMF59-adjuvanted and unadjuvanted vaccine groups only for samples after the third immunization, with P<0.05 (t test). (G)H5N1-HA1–specific affinity of serum IgG after 7 M urea wash in individuals after the third vaccination with either MF59-adjuvanted or unadjuvanted H5N1 subunit vaccine (n = 6). Data are means ± SD of three independent experiments. Differences between groups were examined for statistical significance with Student’s t test. A P value of <0.05 was considered to be significant.