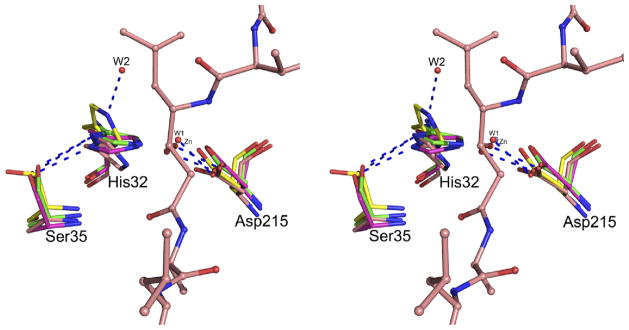
Figure 12.
The active site of HAP in the superimposed structures of apo-HAP (green; PDB ID: 3FNS), HAP-pepstatin A (salmon; PDB ID: 3FNT), HAP–KNI-10006 (magenta; PDB ID: 3FNU), and HAP–KNI-10395 (yellow). The active site residues are shown in stick representation. The nucleophilic water molecules (W1) from the HAP–KNI-10006 and HAP–KNI-10395 structures are shown as red spheres. Another water molecule (W2) from the HAP–KNI-10395 structure is also shown. The Zn2+ ion bound in the active site of apo-HAP, very close behind W1, is gray. Pepstatin A from its HAP complex is shown in ball-and-stick representation. Hydrogen bonds are shown as blue dashed lines.