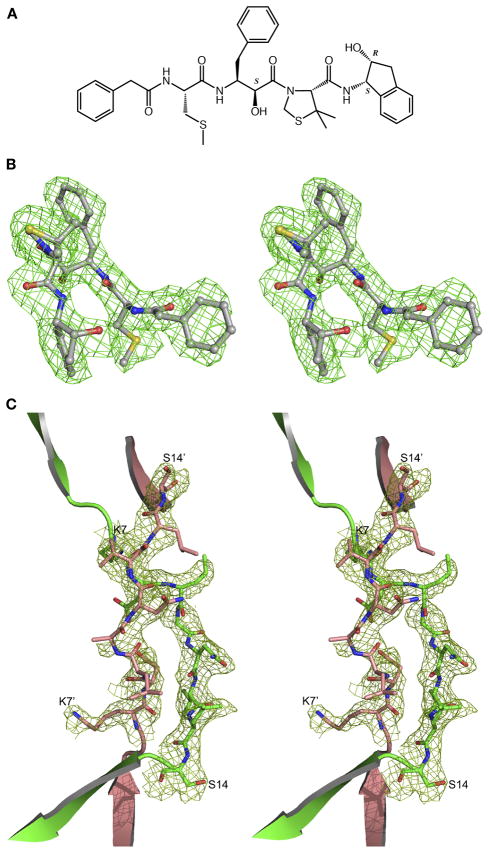
Figure 2.
(A) Chemical structure of KNI-10395. Chiral carbon atoms are labeled S or R. (B) The initial Fo-Fc electron density omitmap contoured at 2.0σ level with the final model superimposed, showing KNI-10395 bound to molecule A of HAP. (C) The Fo-Fc electron density omitmap contoured at 2.0σ level after deleting the domain swap switch region residues 6–14 in molecules A and B (primed) of the HAP–KNI-10395 complex structure, with the final domain swapped polypeptide chain superimposed.