Figure 2.
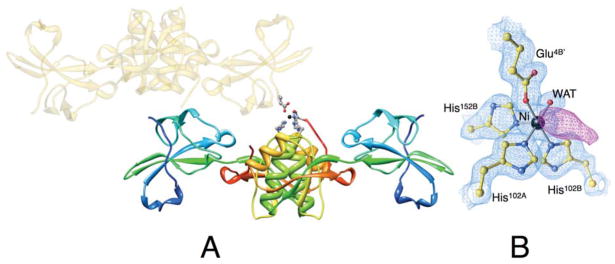
(A) Ribbon scheme of the crystallographic structural model of Ni-HpUreE dimer in the asymmetric unit, with each protomer colored from blue in the proximity of the N-terminal to red at the C-terminus in order to highlight the secondary structure elements along the protein sequence. The symmetry-related dimer that carries the Glu4B′ residue bound to nickel (represented as a black sphere) is shown as transparent gold ribbon. The side chains of the Ni-bound ligands His102A, His102B, His152B, Glu4B′, as well as the solvent molecule, are represented as ball-and-stick models colored according to the CPK color code. Panel B shows a close-up of the coordination environment of the Ni2+ ion together with the 2Fo−Fc electron density map contoured at 1.5 σ (light blue) and the Fo−Fc electron density map contoured at 3.0 σ (violet).