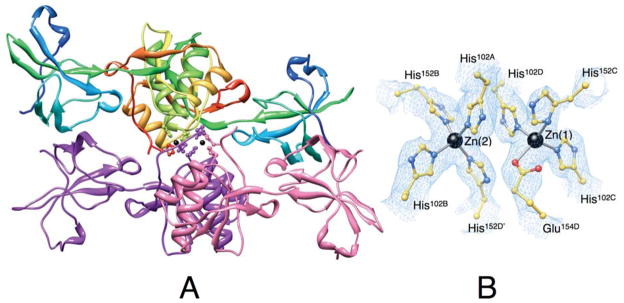
Figure 3.
Ribbon scheme of the crystallographic structural model of Zn-HpUreE dimer of dimers in the asymmetric unit. Each protomer of the dimer on the top is colored from blue in the proximity of the N-terminal to red at the C-terminus in order to highlight the secondary structure elements along the protein sequence. The dimer on the bottom shows the two protomers in different colors. The side chains of the histidine residues binding the Zn2+-ions (shown as black spheres) are represented as ball-and-stick models colored according to their position in the sequence and in the protomer. Panel B shows a close-up of the coordination environment of the Zn2+ ions together with the 2Fo−Fc electron density map contoured at 1.0 σ (light blue).