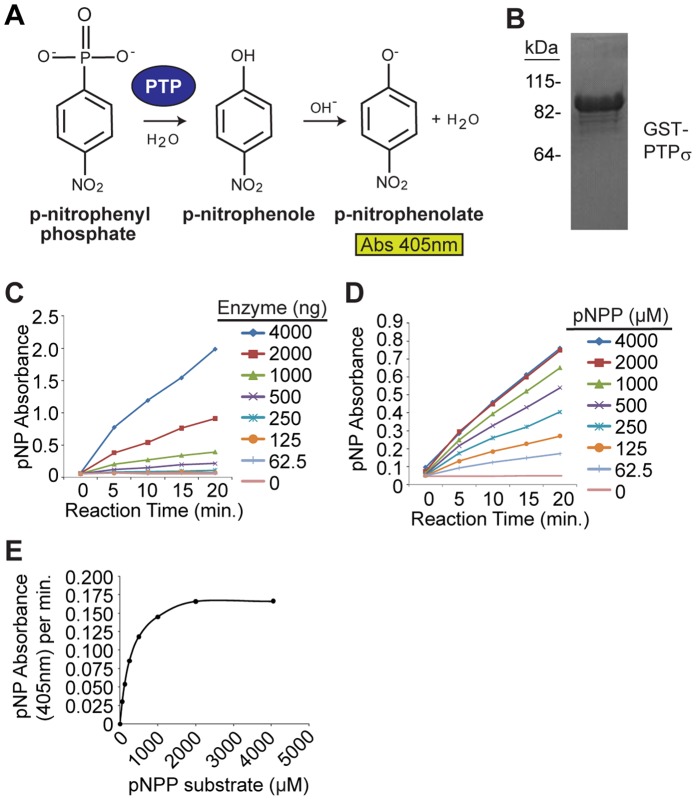
Figure 3. Optimization of biochemical screening conditions for PTPσ inhibition.
(A) Para-nitrophenyl phosphate (pNPP) is a generic phosphatase substrate whose dephosphorylated product, para-nitrophenol (pNP), yields an intense yellow color under alkaline conditions measurable at 405 nm absorbance on a spectrophotometer. (B) 20 µg purified recombinant GST-PTPσ-CTF (C-terminal fragment containing the active sites) protein was resolved by SDS-PAGE and stained with coomassie blue to demonstrate purity. (C) The linear formation of product by various quantities of recombinant GST- PTPσ was observed through time-course reactions. pNPP-phosphatase assays were completed with a saturating dose of 1 mM pNPP. Background-corrected absorbance of dephosphorylated product are plotted by time of reaction. Each plot stems from the quantities of PTPσ indicated in the legend. (D) 2 µg enzyme was chosen from (A) for analysis of activity with varying doses of pNPP substrate. Each plot represents a unique dose of pNPP (indicated in the legend). Background-corrected absorbance of dephosphorylated product are plotted by time of reaction. (E) Initial velocities of PTPσ phosphatase activity (Y-axis; in pNP product formed per minute) were derived from the slopes of the plots in (D) at each of the indicated pNPP substrate concentrations (X-axis). From this, a Km of 250 µM is observed (denoted by dashed line).