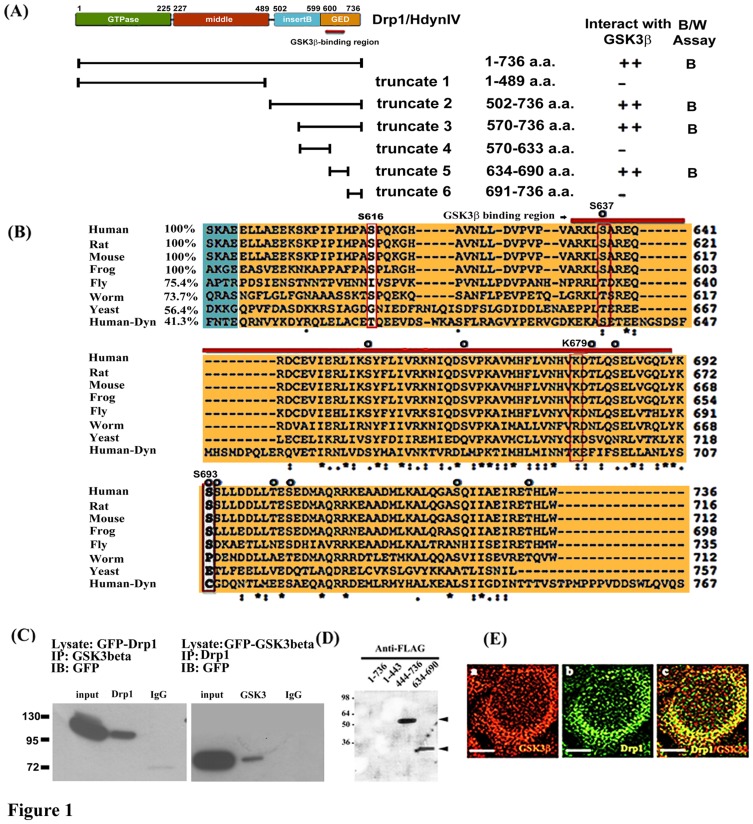
Figure 1. Interaction and co-localization of Drp1/Hydn IV with GSK3beta.
(A) Schematic diagram showing the domain structure of Drp1 as previously described [7] and relative positions of truncated Drp1 constructs used in this study. Yeast two-hybrid assays showing interactions of various Drp1 constructs as bait (pAS2-1) with GSK3beta as prey (pACT-2) as indicated. Blue (B) and white (W) assay was applied for reporter gene beta-galactosidase expression. Strength of interaction was assayed by beta-galactosidase and HIS3 induction as described previously by Hong et al. [5]. (B) Specific binding region of Drp1 with GSK3beta is highly conserved. Multiple alignment of Drp 1 GED domain (colored in orange) was performed using ClustalW2 by which seven Drp1 homologues and human Dynamin 1 were aligned. Note the very high conservation in the GSK3 binding domain (BD, indicated by red bar) in Drp1 but not Dynamin 1 and Ser or Lys residues at positions corresponding to human Drp1 S637, K679, and S693 as indicated by red or white rectangles. Compared to human Drp1, the percentage of amino acid identity corresponding to each BD in GED is listed in front of the sequence. The prospective phosphorylated Ser/Thr residues are indicated by “O”. (C) Total cell lysates were prepared from pEGFP C1-Drp1, pEGPC1-GSK3beta overexpressed 293 cells after transient expression for 24 hours, and subjected to co-immunoprecipitation (IP) assays with anti-GSK3 (left panel), anti-Drp1 (right panel), respectively. The anti-GFP antibody was used to detect the exogenous expressed GFP-tagged proteins in Western blotting (as indicated) which has been described in Materials and Methods. Data are representative of three independent experiments. The IP-Drp1 and IP-GSK3 represents the co-precipitated protein respects to antibodies used (D) The binding fashion of Drp1634–690 and Drp1444–736 fragment with GSK3beta was confirmed by far-Western blotting. Recombinant His-tagged fusion protein, either Drp1 full-length or truncated fragments of Drp1, was separated on 12% SDS polyacrylamide gel. Proteins were transferred into PVDF and incubated with HeLa cell lysate containing FLAG-tagged GSK3beta. Arrowhead indicates binding. (E) Co-localization study was performed by immunocytochemistry staining with confocal fluorescent microscopy as described in Materials and Methods. At 24 hours, 293 cells were stained with anti-GSK3beta antibody to detect endogenous GSK3beta (red, frame a). Frame b is the localization of endogenous Drp1 (green). Frame c is the merged image of the Drp1 and anti- GSK3beta stained cells. Bar = 5 µm.