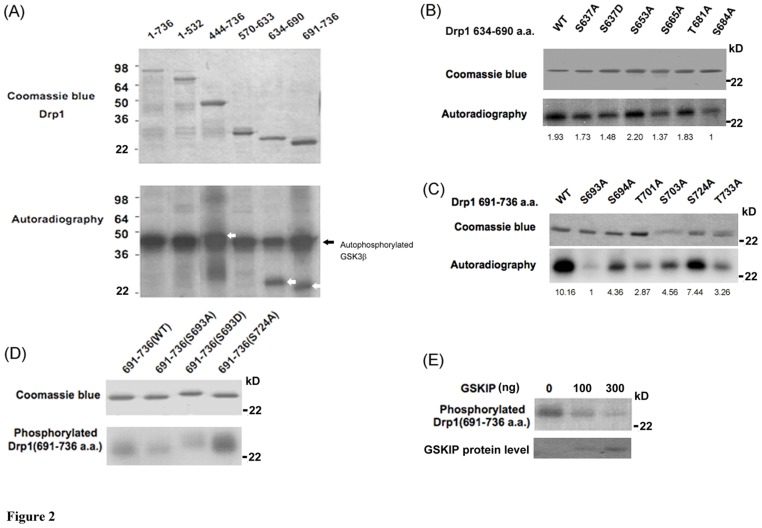
Figure 2. Drp1 is phosphorylated by GSK3beta at Ser693.
Drp1 is phosphorylated by GSK3beta. (A) Purified GSK3beta was used in an in vitro kinase assay to detect whether and which truncated His-tagged Drp1 proteins can act as a substrate to be phosphorylated by GSK3beta. The upper panel is a coomassie blue stained blot showing the His-tagged Drp1 proteins were successfully expressed. The lower panel indicates GSK3beta autophosphorylation (arrow) and phosphorylation of truncated His-Drp1 (empty arrow). (B) In vitro kinase assay to determine the GSK3beta phosphorylation site using site-directed mutagenesis technique. Numbers indicate the mutated residue within the Drp1634–690 fragment. The upper panel is a coomassie blue stained blot showing that His-tagged Drp1634–690 wild-type and mutants were expressed. The lower panel indicates the phosphorylation level with respect to His-tagged Drp1634–690 wild-type and different mutants. (C) Numbers indicate the mutated residue within the Drp1691–736 fragment. The upper panel is a coomassie blue stained blot showing that His-tagged Drp1691–736 wild-type and mutants were expressed. The lower panel indicates the phosphorylation level with respect to His-tagged Drp1691–736 wild-type and different mutants. (D) Both S693A and S693D Drp1 mutants were confirmed to lose their GSK3beta-mediated phosphorylation in vitro. The upper panel is the coomassie blue staining of His-tagged mutated Drp1 fragments. Numbers indicate the mutated residue within the Drp1691–736 fragment. The lower panel indicates the phosphorylation of mutated His-tagged Drp1. (E) The specificity of GSK3beta-mediated phosphorylation was tested on Drp1691–736 fragments. GSK3beta inhibitor, GSKIP, was added in kinase assay with different doses and the effect on the phosphorylation of Drp1691–736 is shown. The coomassie blue image of GSKIP protein as loading/input control was shown in the lower panel.