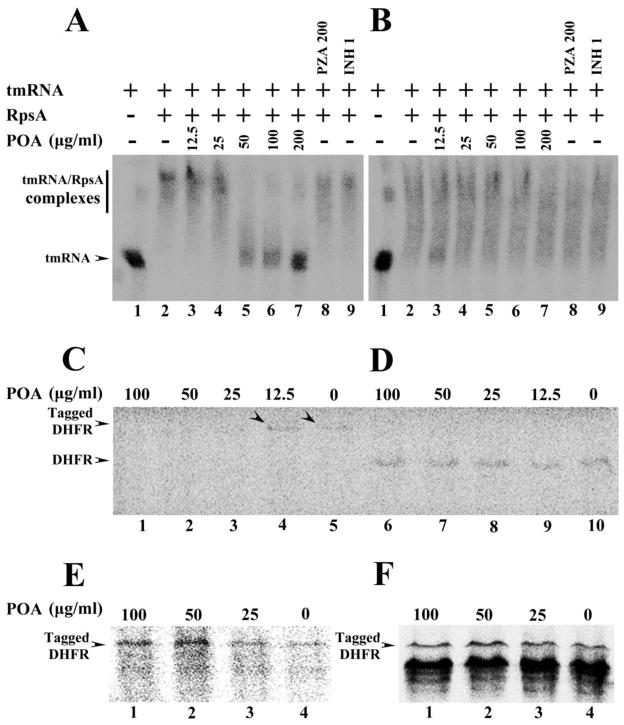
Fig. 3.
(A) Concentration-dependent inhibition of tmRNA binding to wild type M. tuberculosis RpsA by POA (Lanes 2–7). tmRNA from M. tuberculosis was used as RNA alone control (Lane 1). The wild type RpsA interaction with tmRNA was not affected by PZA (200 μg/ml) (Lane 8) or INH (1 μg/ml) (Lane 9). (B) tmRNA had impaired binding to the mutant RpsA (Lane 2), and POA at different concentrations did not inhibit the interaction of the DHM444 mutant RpsA with tmRNA (Lanes 3–7); The mutant RpsA interaction with tmRNA was not affected by PZA (200 μg/ml) (Lane 8) or INH (1 μg/ml) (Lane 9). (C) POA at 100, 50, and 25 μg/ml inhibited trans-translation of the DHFR product in a concentration-dependent manner in the in vitro system that contained ribosomes from M. tuberculosis, tmRNA and recombinant SmpB from M. tuberculosis, template pDHFR-8×AGG rare codons that are required for trans-translation (Lanes 1–5). Arrowheads indicate the trans-translation product DHFR was still present with low concentration of POA at 12.5 μg/ml (Lane 4) or in the absence of POA (Lane 5). POA at different concentrations did not inhibit canonical translation in in vitro translation system using ribosomes from M. tuberculosis, template pDHFR with stop codon (D, Lanes 6–10), nor the trans-translation of DHFR using ribosome from M. smegmatis (E, Lanes 1–4), or using ribosome from E. coli (F, Lanes 1–4) in the trans-translation system that contained tmRNA and recombinant SmpB from M. tuberculosis, template pDHFR-8×AGG rare codons.