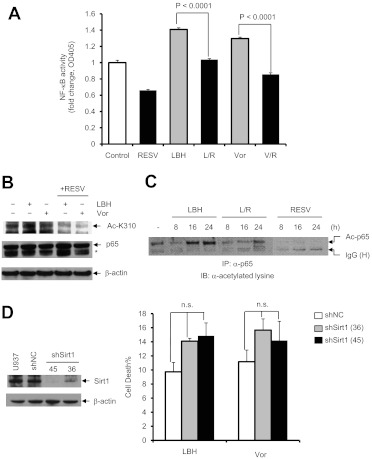
Fig. 3.
Resveratrol inhibits HDACI-induced RelA/p65 acetylation and NF-κB activation. A, U937 cells were incubated with 50 μM resveratrol (RESV) with or without 15 nM LBH-589 (LBH) or 1.5 μM vorinostat (Vor) for 8 h, after which NF-κB activity was determined with an ELISA-based, p65-specific, NF-κB activity assay. R, resveratrol; L, LBH-589; V, vorinostat. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. B, U937 cells were treated for 24 h as described for A, and Western blot analysis was performed with antibodies specifically recognizing Lys310-acetylated p65. Total p65 was probed for comparison. *, nonspecific bands. C, cells were harvested and lysed at the indicated times, and 200 μg of protein was subjected to immunoprecipitation (IP) with anti-p65 antibody, followed by Western blot analysis with antilysine antibody. IB, immunoblotting; Ac, acetylated; IgG (H), IgG heavy chain. D, U937 cells were stably transfected with constructs encoding shRNA specifically targeting Sirt1 (shSirt1) or a scrambled sequence as negative control (shNC). Western blot analysis was performed to determine the down-regulation of Sirt1 protein in two shSirt1 clones (designed 36 and 45), compared with parental U937 and shNC-transfected cells. Cells were then exposed to 15 nM LBH-589 or 1.5 μM vorinostat for 48 h, after which cell death was monitored through 7-aminoactinomycin D staining and flow cytometry. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. n.s., not significant (P > 0.05). For Western blot analysis, each lane was loaded with 30 μg of protein; blots were stripped and reprobed for β-actin to ensure equivalent loading and transfer.