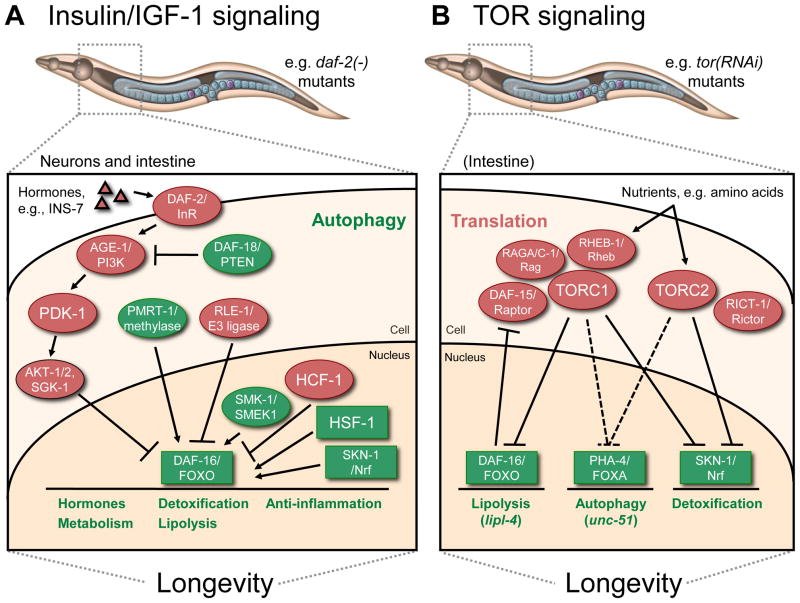
Figure 1. Overview of insulin/IGF-1 (A) and TOR (B) signaling in C. elegans aging.
A. In daf-2/InR insulin-receptor mutants, insulin-like peptides (e.g., INS-7) secreted from neurons reach intestinal cells and trigger the canonical insulin-signaling pathway, which prevents DAF-16/FOXO from entering the nucleus. Other mechanisms of DAF-16/FOXO regulation include ubiquitination (RLE-1/E3 ligase) and arginine methylation (PMRT-1/methylase). Nuclear-localized DAF-16/FOXO activity is enhanced by the action of SMK-1/SMEK and HSF-1, and is inhibited by HCF-1. The transcription factor SKN-1/Nrf is also required for longevity in daf-2/InR mutants. Collectively, these factors transcriptionally regulate multiple output processes as noted. Autophagy is another cellular process required for daf-2/InR mutants to live long. It is not yet known whether autophagy is a transcriptionally regulated process in daf-2/InR mutants.
B. TOR responds to nutrients and functions in two different complexes, TORC1 and TORC2. In analogy with mammalian studies, TORC1 is thought to interact with DAF-15/Raptor as well as Rag GTPases like RAGA-1, RAGC-1, and RHEB-1/Rheb. TORC1 and TORC1 specifically impair the activity of DAF-16/FOXO and SKN-1/Nrf, whereas it is not yet clear which of the two TOR complexes regulates PHA-4/FOXA (indicated by dashes lines). By using these transcription factors, TOR inhibits the expression of at least certain lipolysis-, autophagy-, and detoxification-associated genes. Listed as a cytoplasmic process, TOR signaling also likely modulates aging through a general suppression of translation. While the intestine has been linked to the longevity mediated by TORC1, the specific tissue requirements for TOR-dependent effects on aging have not yet been systematically investigated.
Factors with longevity-promoting effects are in green and those with lifespan-limiting effects are in red. Transcription factors are in boxes. See text for details.