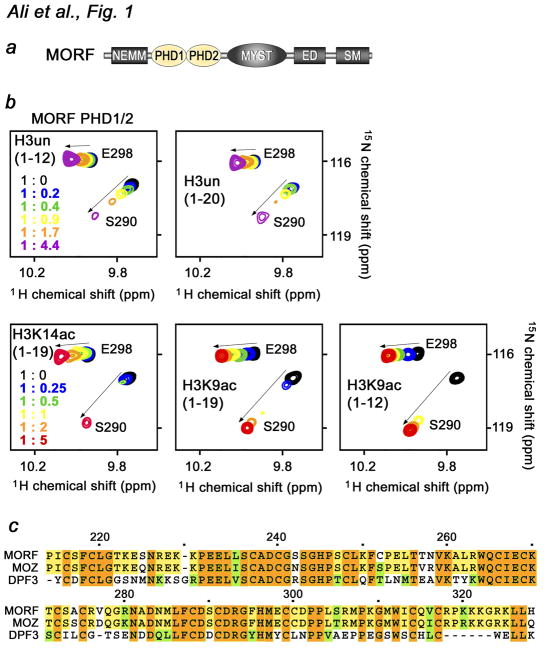
Fig. 1.
The MORF PHD1/2 fingers bind histone H3. (a) Architecture of MORF. The tandem PHD1/2 fingers are colored yellow. (b) Superimposed 1H,15N HSQC spectra of 0.1 mM MORF PHD1/2, collected as indicated H3 peptides were separately titrated in. The spectra are color-coded according to the protein-peptide ratio (inset). (c) Alignment of the PHD1/2 sequences: absolutely, moderately and weakly conserved residues are colored brown, yellow and green, respectively. Each tenth residue of MORF PHD1/2 is marked by a dot and labeled.