Fig. P1.
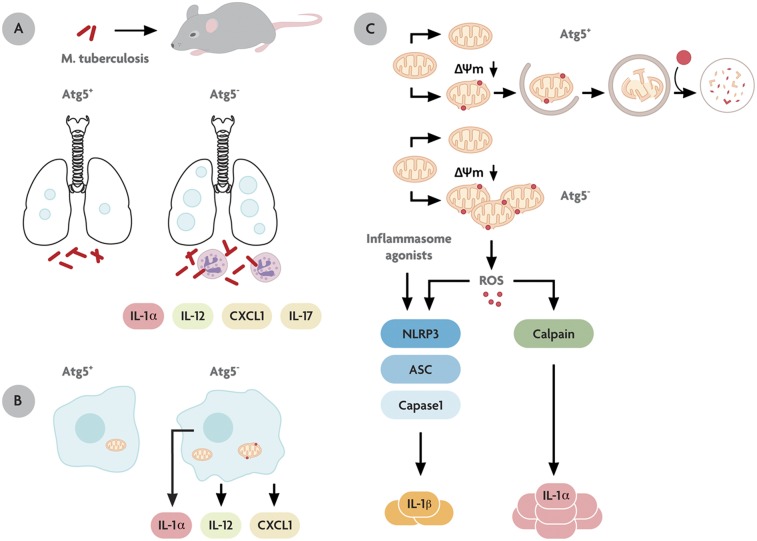
Autophagy protects against M. tuberculosis infection and excessive inflammation in vivo. (A) The M. tuberculosis-infected lung in mice defective for autophagy (Atg5−) shows increased pathology, necrosis, neutrophil infiltration, and excessive production of the proinflammatory cytokines IL-1α, IL-12, CXCL1, and IL-17. (B) Macrophages defective for autophagy (Atg5−) secrete proinflammatory cytokines IL-1α, IL-12, and CXCL1 in a cell-autonomous fashion. (C) Specialized organelles called “autophagosomes” (double-membrane organelles enveloping mitochondria) remove invading microbes from the eukaryotic cytoplasm or disused organelles such as depolarized mitochondria. Cells that cannot continuously remove depolarized (Δψm↓) mitochondria through autophagy are prone to IL-1α hypersecretion caused by reactive oxygen intermediates (ROS; small red dots). This state is known to induce inflammasome (composed of NLRP3, ASC, and caspase 1) activation and IL-1β secretion; however, IL-1α hypersecretion differs, because it uses a calpain-dependent pathway independently of the inflammasome.