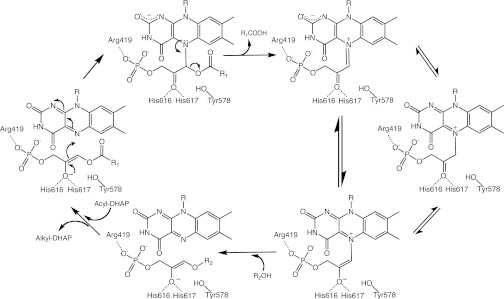
Fig. 1.
Mechanistic proposal for the ADPS reaction. The acylDHAP substrate is shown in its enolic form. The crucial covalent intermediate can have different tautomeric forms. The exact protonation states of several titratable groups purportedly involved in the reaction (Tyr578, His616-His617, N1 and N5 of the flavin, oxygens of DHAP, fatty acid product, and fatty alcohol substrate) are not firmly defined and are likely to change during the reaction. The data do not rule out adduct formation on C4a rather than on N5 of the flavin (see Fig. 4C, Right for atomic numbering); however, a C4a adduct intermediate would be difficult to reconcile with the subsequent fatty acid elimination step. The predicted negative charge of the covalent adduct can be stabilized by a network of H-bonding interactions involving protein backbone atoms, an ordered water molecule, and the N1 and O2 atoms of the flavin, as shown in Fig. S2A.