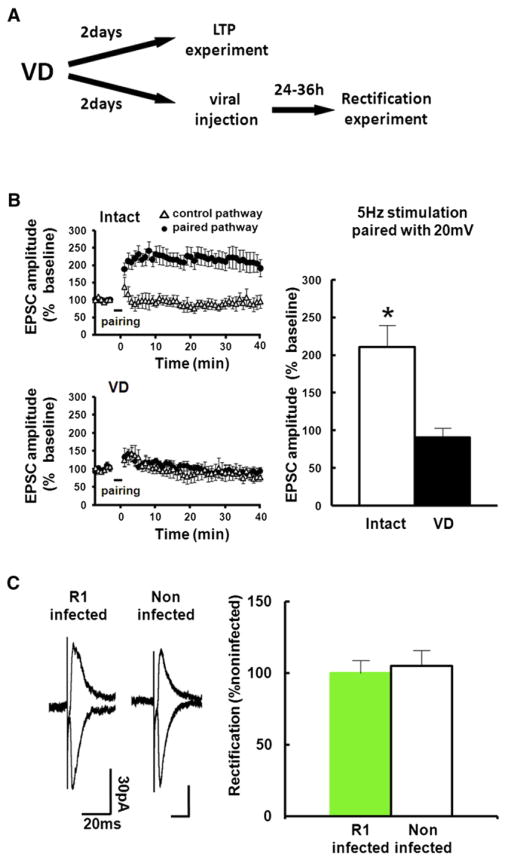
Figure 2. VD Procedure for 2 Days Occludes LTP in the Barrel Cortex.
(A) Experimental protocol. We sutured both eyes at P21. For LTP experiment, acute brain slices were prepared at P23. Then LTP at layer 4-2/3 synapses of the barrel cortex was induced by pairing postsynaptic depolarization (+20 mV) with presynaptic stimulation (5 Hz, 90 s). For the rectification experiment, we microinjected GFP-GluR1 expressing virus at P23, and whole-cell recordings were performed at P25.
(B) Recordings were maintained for at least 40 min after pairing. (Left) The EPSC amplitude was normalized to the average baseline amplitude before pairing. Paired pathways (black circle) and control pathways (triangle) of intact and VD animals are shown. (Right) Mean amplitude between 30 and 40 min after induction was normalized to base line amplitude. While LTP was successfully induced in intact rats, LTP induction was prevented in animals with 2 days of VD, indicating that 2 days of VD saturates synaptic AMPARs contents and occludes LTP. Intact: n = 7, VD: n = 7. Scale bars are as indicated. *Statistical difference (Student’s t test). Error bars indicate ± SEM.
(C) (Left) Synaptic responses from layer 4 to layer 2/3 pyramidal neurons at P25 infected with GFP-GluR1 expressing virus at P23 and noninfected neurons of juvenile rats exposed to VD from P21. Note no difference of rectification between infected and noninfected neurons, indicating no further delivery of GluR1 after 2 days of VD. (Right) Graph of average RI of neurons expressing GFP-GluR1 (green), normalized to RI value of nearby noninfected cells (white). n = 7. Scale bars are as indicated. Note that there was no statistical difference.
Error bars indicate ± SEM.
See also Figure S2.