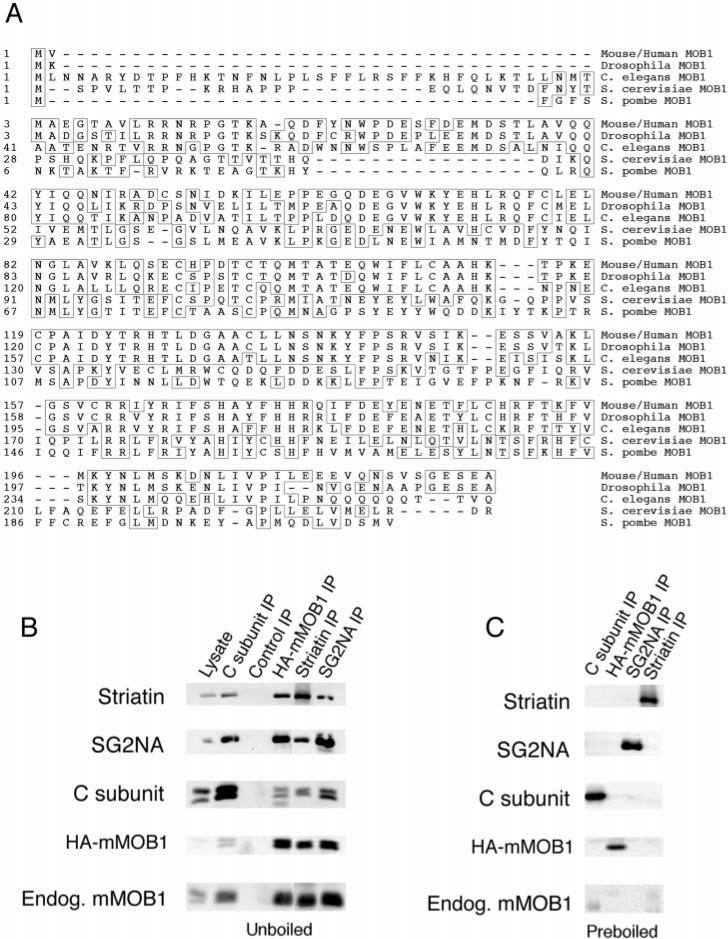
Fig. 1. SG2NA-PP2A and striatin-PP2A form stable complexes with mammalian class II MOB1.
A, amino acid sequence alignment of class II MOB1 from mouse/human, Drosophila, and C. elegans with S. cerevisiae and S. pombe MOB1. Human and mouse MOB1 are 100% identical at the amino acid level. Amino acids matching the consensus sequence are boxed. B, a whole cell lysate and the indicated immunoprecipitations (IP) were prepared from NIH3T3 cells that stably express HA-tagged mMOB1. Immune complexes were analyzed by SDS-PAGE, transferred to nitrocellulose, and sequentially probed by immunoblotting. Striatin, SG2NA, PP2A C subunit, HA-mMOB1, and endogenous (Endog.) mMOB1 were detected in 12CA5 immunoprecipitations of HA-mMOB1 but not in a control immunoprecipitation. Also, HA-mMOB1 and endogenous mMOB1 were detected in PP2A (1d6), striatin, and SG2NA immunoprecipitations but not in control immunoprecipitations. Control immunoprecipitations were performed using the 7-34-1 monoclonal antibody (American Type Culture Collection) directed against major histocompatibility complex class I swine leukocyte antigen. C, as an additional control, some lysates were preboiled in 0.5% SDS and 5 mm β-mercaptoethanol to disrupt complexes, and then parallel immunoprecipitations were prepared. The absence of co-immunoprecipitating proteins from preboiled lysates indicates that co-immunoprecipitations were not due to cross-reactive antibodies.