Abstract
Successful treatment of soft-tissue sarcomas is highly dependent on total tumour resection coupled with adjuvant radiation therapy to achieve local control and decrease recurrence. Reconstruction of soft-tissue defects after resection aims to cover vital structures, while providing enough stable tissue to withstand adjuvant brachytherapy treatment. In the present study, pedicled myocutaneous flaps were used as a vital adjunct in the treatment of soft-tissue sarcoma, and our experience with 2 such patients is described. The flaps served to reconstruct large three-dimensional defects while providing stable coverage over brachytherapy hardware to allow for delivery of radiation in the immediate postoperative period. Pedicled locoregional myocutaneous flaps provide a safe, easy, and reliable reconstructive technique in the treatment of soft-tissue sarcoma.
Keywords: Sarcoma, pedicled myocutaneous flaps, wound coverage, brachytherapy, radiation, adjuvant cancer therapy
1. INTRODUCTION
Despite being rare tumours that represent approximately 1% of all adult cancers1, soft-tissue sarcomas are associated with poor clinical outcomes. Among these patients, 10%–30% experience local recurrence, and 30%–45% experience distant failure2. Wide resection with negative margins, in conjunction with postoperative interstitial brachytherapy, improves local control2,3. With the potential for large curative resections, a need arises for durable reconstruction4.
The use of tissue transfers after neoadjuvant radiation has been extensively described5,6, but few reports have examined such techniques in the context of intraoperative brachytherapy catheter placement, with radiation initiated in the early postoperative period. At our institution, 2 patients underwent resection of soft-tissue sarcomas with immediate reconstruction using pedicled musculocutaneous flaps and were treated with “immediate” brachytherapy. Both patients commenced brachytherapy no later than the 4th postoperative day. Although use of these regional myocutaneous flaps has been extensively described for soft-tissue reconstructions after sarcoma resection, few reports have examined their utility in early postoperative brachytherapy7–13.
2. CASE DESCRIPTIONS
2.1. Case 1
A 64-year-old white man presented with an enlarging left shoulder mass [Figure 1(A)]. Imaging revealed a 4.8×4.0×5.2 cm3 mass located in the deltoid muscle. Subsequent biopsy identified a pleomorphic malignant fibrous histiocytoma with undifferentiated high-grade histology. Metastatic work-up revealed no distant disease.
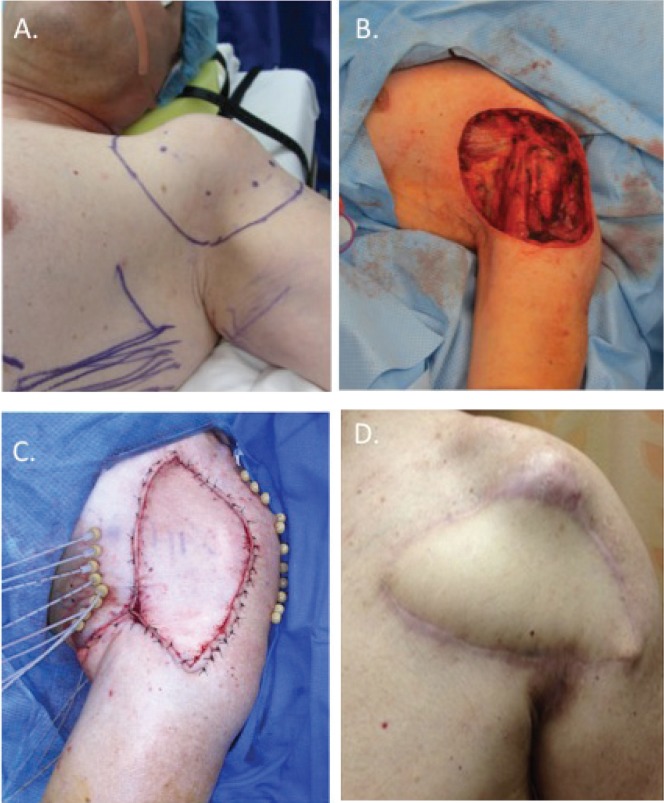
FIGURE 1.
(A) Soft-tissue sarcoma in the left shoulder, involving the deltoid and pectoralis major muscle. (B) Post-resection surgical defect, measuring 14×20 cm2, demonstrating exposed left shoulder capsule. (C) Brachytherapy catheters (n = 11) positioned intraoperatively above the tumour bed and below the latissimus dorsi myocutaneous flap. (D) At 6 months postoperatively, the flap has healed with minimal complications.
An interdisciplinary team approach consisting of a surgical oncologist, a plastic surgeon, and a radiation oncologist coordinated single-stage therapy for this patient. Resection of the tumour en bloc, together with the anterior portion of the deltoid and the lateral portion of the pectoralis major muscle, was performed [Figure 1(B)]. Eleven brachytherapy catheters were positioned at 1-cm intervals across a tumour bed measuring 14×20 cm2 [Figure 1(C)]. Coverage of the exposed shoulder capsule and brachytherapy catheters was accomplished using a rotational latissimus dorsi musculocutaneous flap based on the thoracodorsal neurovascular pedicle.
The patient commenced brachytherapy treatment on the 4th postoperative day, receiving a total of 3330 cGy, given in 9 fractions over 4 days. No immediate postoperative complications were noted. One small (<1 cm2) area of non-coverage along the inferior aspect of the wound remained, which healed by secondary intent 2 weeks postoperatively. After 8 months of follow-up, the flap had completely healed into its host location [Figure 1(D)].
2.2. Case 2
A 63-year-old Hispanic man presented with recurrence of a grade 2/3 myxoid chondrosarcoma after initial treatment with left internal hemipelvectomy combined with adjuvant chemotherapy and radiation [Figure 2(A)]. Diagnostic imaging revealed a 7.4×8.8×7.4 cm3 mass anterior to the left femoral head, and a second mass measuring 3.6×5.9×3.6 cm3 superior to the left gluteus maximus muscle. As in case 1, a coordinated single-stage approach was used.
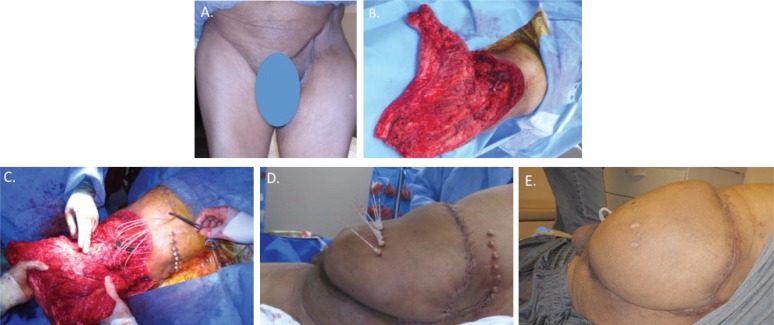
FIGURE 2.
(A) Recurrent chondrosarcoma of the left hip region after prior left internal hemipelvectomy. (B) Post left-hindquarter resection, with preservation of posterior thigh flap based on the remaining gluteal muscles. (C) Insertion of brachytherapy catheters overlying the tumour bed. (D) Flap inset to cover the vital structures of the lower trunk and the brachytherapy catheters (posterior view). (E) At 1 month postoperatively, the flap has healed and only a single drain remains (posterior view).
Resection consisted of a left-hindquarter external pelvic resection [Figure 2(B)], which resulted in a secondary defect measuring 800 cm2, with exposed intraperitoneal structures. Ten brachytherapy catheters were positioned across the tumour bed at 1-cm intervals [Figure 2(C)]. A gluteus maximus myocutaneous flap with an extended skin paddle derived from the left buttock and posterior thigh was used to provide coverage over exposed ureter, bladder, lower lumbar spine, and left hemipelvis [Figure 2(D)].
Brachytherapy treatment began on the 3rd postoperative day. The patient received a total radiation dose of 1500 cGy, given in 5 fractions over 7 days. No immediate postoperative complications were noted. At 6 months of follow-up, the flap had completely healed without signs of wound breakdown [Figure 2(E)].
Unfortunately, shortly thereafter, the patient experienced a recurrence in close proximity to the original site of resection.
3. DISCUSSION
In sarcoma treatment, adjuvant radiation improves tumour control and provides the opportunity for limb salvage. Brachytherapy is comparably efficacious to external-beam radiation therapy (ebrt)14, but allows for more-targeted delivery with higher radiation doses, shorter treatment duration, and fewer treatment courses15. It also permits the near-immediate postoperative delivery of radiation to achieve microscopic tumour ablation. Furthermore, brachytherapy minimizes collateral tissue damage because of more selective tumour bed targeting, thus facilitating subsequent reconstruction. Despite those advantages, brachytherapy does not seem to be as effective as intensity-modulated radiation therapy for the treatment of low-grade sarcomas compared with high-grade tumours, as some reports have suggested16,17. However, ebrt and intensity-modulated radiation therapy do miss a critical therapeutic window because delivery is often delayed for 4–6 weeks after tumour resection to allow for complete wound healing18.
Notwithstanding the therapeutic potential of brachytherapy during the immediate perioperative period, concerns over wound complications abound. Wound dehiscence, infection, seroma, and hematoma are radiation-induced complications that can threaten the viability of the reconstruction and can delay adjuvant radiation treatment and chemotherapy18–20. Moreover, compared with ebrt, brachytherapy has been shown to increase the rate of perioperative wound complications despite its minimization of long-term damage to the surrounding viable tissues. To address those concerns, clinicians have typically delayed brachytherapy to the 5th postoperative day both to minimize complications and to treat residual disease in a time-efficient manner18.
Current reconstructive options capable of meeting the needs of brachytherapy are limited. Primary wound closure alone often results in insufficient wound coverage. Because tension-free wound approximation is often not possible, large defects are not amenable to primary closure without tissue expander devices to stretch nearby tissue. However, tissue expanders necessitate significant delays impractical for immediate treatment, especially if brachytherapy catheters are a consideration. Expanders are also associated with frequent complications in head-and-neck and extremity reconstruction21–23. Furthermore, Shibata et al. found that, in the setting of adjuvant radiation, wound healing complications were more frequent for wounds closed by primary approximation than for those closed using vascularized tissue flaps24. To date, no study has evaluated the same association when brachytherapy alone is used.
Skin grafts may be used for wound coverage, but they do not provide adequate bulk for deep, three-dimensional wounds. Given that survival is highly dependent on contact with the wound surface, skin grafts also cannot offer sufficient protection for underlying brachytherapy catheters. Similarly, grafting skin onto isolated muscle flaps is often suboptimal, because skin grafts are prone to fail in the setting of intervening brachytherapy catheters or of a radiated and compromised wound bed. Local skin flaps are tenuous too, because their randomly-based blood supply makes them insufficiently perfused and thus vulnerable to postoperative radiation. In contrast, musculocutaneous flaps offer robust reconstruction for coverage of complex three-dimensional defects, and they are also tolerant of brachytherapy.
Free-tissue transfer has been extensively described in the literature. It offers a useful reconstructive option after sarcoma resection, but its use in the setting of brachytherapy has inherent drawbacks15,25,26. Being highly sensitive to anastomotic complications in the immediate postoperative period, free-tissue transfers rely on meticulous microvascular technique and careful flap in-setting. Brachytherapy catheters beneath a free-tissue flap could exert distortional forces resulting in flap ischemia or direct mechanical pressure on the anastomosis. Despite success rates in excess of 95% in some centres, cumbersome postoperative monitoring and a low threshold for potential anastomotic revision may preclude timely delivery of radiation therapy. Additionally, the implications for total flap loss in the event of anastomotic complications equate to total exposure of the underlying wound bed, brachytherapy hardware, and vital structures. A recent study by Chao et al.27 suggested a lack of recipient-site complications after free-tissue transfer for reconstruction after sarcoma resection. Various sarcomas were included in the analysis, but the study examined complications in the setting of adjuvant and neoadjuvant ebrt that did not involve the placement of hardware beneath the flaps.
The present series provides two examples of common pedicled myocutaneous flaps, used in the immediate reconstruction of sarcoma defects, in which postoperative brachytherapy was promptly given. Previous studies have reported on the tolerance of pedicled myocutaneous flaps to brachytherapy. Spierer et al.26 demonstrated a 6% 5-year re-operation rate secondary to wound complications when initiating brachytherapy 7 days after reconstruction. Goel et al.4 and Lee et al.28 reached similar findings in their respective investigations. Those results could, in part, be attributable to the significantly lower dose of radiation delivered per fraction of brachytherapy (<40 cGy) compared with that delivered by ebrt (>35 Gy per fraction). Adding to a growing body of literature, our study demonstrates the ability of pedicled myocutaneous flaps to tolerate postoperative adjuvant brachytherapy and extends those observations to include the immediate postoperative period at days 3–4.
As a reconstructive option after sarcoma resection, pedicled musculocutaneous flaps offer several advantages in the setting of postoperative brachytherapy. They bring much-needed perfusion to an already compromised wound bed vulnerable to imminent irradiation29. Postoperative monitoring of the flaps is less complicated: in contrast to free-tissue transfers, pedicled flaps do not require frequent flap checks, stringent positioning protocols, or postoperative anticoagulation to maintain anastomotic patency. Partial flap loss is possible, but total flap loss requiring additional salvage procedures is less likely than with a free-tissue transfer.
Concerns over tumour recurrence because of distant iatrogenic implantation during oncologic resection do exist. However, sarcoma recurrence at donor-tissue sites after reconstruction are a rare occurrence. They have been described only in select case reports—and should not preclude reconstruction30. The effect of donor-site radiation on the incidence of distant sarcoma recurrence has yet to be examined. However, the use of meticulous surgical technique can prevent tumour seeding in micropositive resections when negative margins cannot be obtained. As such, studies have confirmed the use and safety of immediate reconstruction after sarcoma resection26,28. Ultimately, the great familiarity of most plastic surgeons with regional musculocutaneous flaps makes such flaps universally applicable and quite useful, especially in centres in which microvascular expertise or resources are limited.
4. CONCLUSIONS
Pedicled myocutaneous flaps are a viable addition to the reconstructive armamentarium for post-sarcoma resection defects. Their tolerance to early postoperative brachytherapy permits prompt initiation of treatment. The present series also illustrates the utility of an interdisciplinary team approach to the provision of comprehensive patient care.
5. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
6. REFERENCES
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–11. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Llacer C, Delannes M, Minsat M, et al. Low-dose intraoperative brachytherapy in soft tissue sarcomas involving neurovascular structure. Radiother Oncol. 2006;78:10–16. doi: 10.1016/j.radonc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MF, Hilaris B, Shiu MH, et al. Local recurrence in adult soft-tissue sarcoma. A randomized trial of brachytherapy. Arch Surg. 1987;122:1289–93. doi: 10.1001/archsurg.1987.01400230075014. [DOI] [PubMed] [Google Scholar]
- 4.Goel V, Goel A, Gupta N, Bhamre S. Flap reconstruction and interstitial brachytherapy in nonextremity soft tissue sarcoma. J Cancer Res Ther. 2007;3:105–7. doi: 10.4103/0973-1482.34690. [DOI] [PubMed] [Google Scholar]
- 5.Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93:980–7. doi: 10.1097/00006534-199404001-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bell RS, Mahoney J, O’Sullivan B, et al. Wound healing complications in soft tissue sarcoma management: comparison of three treatment protocols. J Surg Oncol. 1991;46:190–7. doi: 10.1002/jso.2930460314. [DOI] [PubMed] [Google Scholar]
- 7.Kim JS, Lee JS, Yoon JO, Park JB. Reconstruction of the shoulder region using a pedicled latissimus dorsi flap after resection of soft tissue due to sarcoma. J Plast Reconstr Aesthet Surg. 2009;62:1215–18. doi: 10.1016/j.bjps.2007.12.079. [DOI] [PubMed] [Google Scholar]
- 8.Behnam AB, Chen CM, Pusic AL, et al. The pedicled latissimus dorsi flap for shoulder reconstruction after sarcoma resection. Ann Surg Oncol. 2007;14:1591–5. doi: 10.1245/s10434-006-9292-5. [DOI] [PubMed] [Google Scholar]
- 9.Hernanz F, Sanchez S, Perez–Cerdeira MP, Redondo–Figuero C. Long-term results of breast conservation and immediate volume replacement with myocutaneous latissimus dorsi flap. World J Surg Oncol. 2011;9:159. doi: 10.1186/1477-7819-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perdikis G, Koonce S, Collis G, Eck D. Latissimus dorsi myocutaneous flap for breast reconstruction: bad rap or good flap? Eplasty. 2011;11:e39. [PMC free article] [PubMed] [Google Scholar]
- 11.Kishi K, Nakajima H, Imanishi N, Nakajima T. Extended split superior gluteus maximus musculocutaneous flap and reconstruction after resection of perianal and lower gluteal hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2009;62:1081–6. doi: 10.1016/j.bjps.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen TH. Bilateral gluteus maximus V–Y advancement musculocutaneous flaps for the coverage of large sacral pressure sores: revisit and refinement. Ann Plast Surg. 1995;35:492–7. doi: 10.1097/00000637-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kim JT, Kim YH, Naidu S. Perfecting the design of the gluteus maximus perforator-based island flap for coverage of buttock defects. Plast Reconstr Surg. 2010;125:1744–51. doi: 10.1097/PRS.0b013e3181cb675f. [DOI] [PubMed] [Google Scholar]
- 14.Alektiar KM, Velasco J, Zelefsky MJ, Woodruff JM, Lewis JJ, Brennan MF. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2000;48:1051–8. doi: 10.1016/S0360-3016(00)00753-7. [DOI] [PubMed] [Google Scholar]
- 15.Papagelopoulos PJ, Mavrogenis AF, Mastorakos DP, Vlastou C, Vrouvas J, Soucacos PN. Free vascularised tissue transfer and brachytherapy for soft-tissue sarcomas of the extremities. Injury. 2008;39(suppl 3):S83–9. doi: 10.1016/j.injury.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–5. doi: 10.1200/JCO.1994.12.6.1150. [DOI] [PubMed] [Google Scholar]
- 17.Alektiar KM, Brennan MF, Singer S. Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer. 2011;117:3229–34. doi: 10.1002/cncr.25882. [DOI] [PubMed] [Google Scholar]
- 18.Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210:93–9. doi: 10.1097/00000658-198907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbeit JM, Hilaris BS, Brennan MF. Wound complications in the multimodality treatment of extremity and superficial truncal sarcomas. J Clin Oncol. 1987;5:480–8. doi: 10.1200/JCO.1987.5.3.480. [DOI] [PubMed] [Google Scholar]
- 20.Emory CL, Montgomery CO, Potter BK, Keisch ME, Conway SA. Early complications of high-dose-rate brachytherapy in soft tissue sarcoma: a comparison with traditional external-beam radiotherapy. Clin Orthop Relat Res. 2012;470:751–8. doi: 10.1007/s11999-011-2106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713–21. doi: 10.1016/S0360-3016(00)01402-4. [DOI] [PubMed] [Google Scholar]
- 22.Meland NB, Loessin SJ, Thimsen D, Jackson IT. Tissue expansion in the extremities using external reservoirs. Ann Plast Surg. 1992;29:36–9. doi: 10.1097/00000637-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hurvitz KA, Rosen H, Meara JG. Pediatric cervicofacial tissue expansion. Int J Pediatr Otorhinolaryngol. 2005;69:1509–13. doi: 10.1016/j.ijporl.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Shibata D, Hyland W, Busse P, et al. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33–7. doi: 10.1007/s10434-999-0033-4. [DOI] [PubMed] [Google Scholar]
- 25.Aflatoon K, Manoso MW, Deune EG, Frassica DA, Frassica FJ. Brachytherapy tubes and free tissue transfer after soft tissue sarcoma resection. Clin Orthop Relat Res. 2003:248–53. doi: 10.1097/01.blo.0000093886.12372.74. [DOI] [PubMed] [Google Scholar]
- 26.Spierer MM, Alektiar KM, Zelefsky MJ, Brennan MF, Cordiero PG. Tolerance of tissue transfers to adjuvant radiation therapy in primary soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys. 2003;56:1112–16. doi: 10.1016/S0360-3016(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 27.Chao AH, Chang DW, Shuaib SW, Hanasono MM. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast Reconstr Surg. 2012;129:675–82. doi: 10.1097/PRS.0b013e3182412a39. [DOI] [PubMed] [Google Scholar]
- 29.Ma CH, Tu YK, Wu CH, Yen CY, Yu SW, Kao FC. Reconstruction of upper extremity large soft-tissue defects using pedicled latissimus dorsi muscle flaps—technique illustration and clinical outcomes. Injury. 2008;39(suppl 4):67–74. doi: 10.1016/j.injury.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Hughes TM, Thomas JM. Sarcoma metastases due to iatrogenic implantation. Eur J Surg Oncol. 2000;26:50–2. doi: 10.1053/ejso.1999.0740. [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, Cordeiro PG, Mehrara BJ, et al. Reconstruction after soft tissue sarcoma resection in the setting of brachytherapy: a 10-year experience. Ann Plast Surg. 2004;52:486–91. doi: 10.1097/01.sap.0000122649.64350.e3. [DOI] [PubMed] [Google Scholar]