Figure 1 .
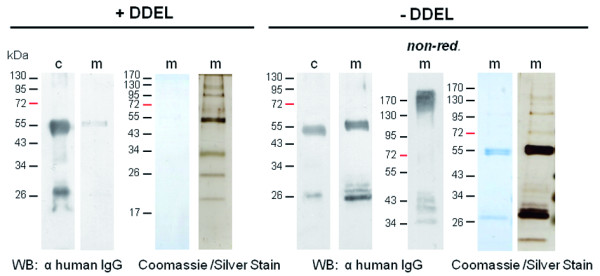
Analyses on antibody secretion byP. tricornutum. Light and heavy chain of the human IgG antibody CL4mAb against the Hepatitis B Virus surface protein were expressed in P. tricornutum for two days either with (+DDEL) or without an ER-retention signal (−DDEL). Proteins of the cellular fraction (c) and the cell-free medium (m) were subsequently separated by gel electrophoresis and analyzed by Western Blot and Coomassie/Silver Staining. In cells expressing an ER-retention signal hardly any antibody is detected in the medium, whereas the deletion of the retention signal leads to efficient antibody secretion. Under non-reduced conditions a high molecular weight signal corresponding to the fully assembled antibody (consisting of two heavy and two light chains) is detected. For Western Blot analyses/Coomassie and Silver Staining proteins of 15 ml/30 ml cell-free medium were concentrated and precipitated. 10 μg protein of the cellular fractions was loaded.
