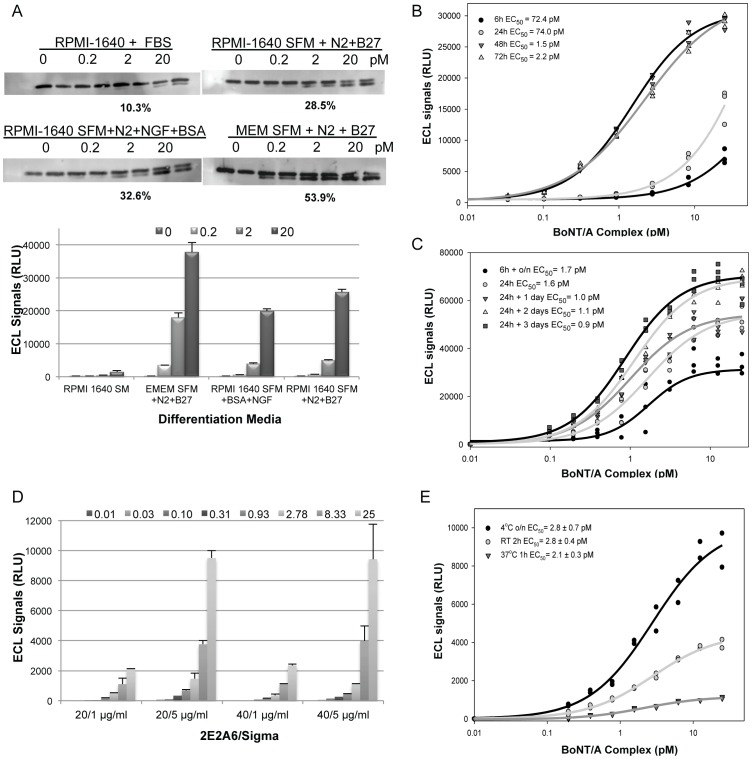
Figure 5. Optimization the SiMa CBPA.
A. Optimization of differentiation medium. SiMa cells were plated in RPMI-1640 with FBS (SM), RPMI-1640 serum-free medium (SFM) supplemented with N2 and B27, RPMI-1640 SFM with N2, BSA and NGF, or EMEM SFM with N2 and B27 for three days. Cells were treated with BoNT/A at 0.2, 2 and 20 pM for 24 h and incubated for 48 h. Lysates were analyzed by WB and the percent cleavage at 2 pM is shown. The same lysates were analyzed in the ECL-ELISA confirming that the EMEM SFM with N2 and B27 supplements was optimal for differentiation (Error bars = std. dev.). Clear signal over background was detected at 0.2 pM. B. Optimization of differentiation time. SiMa cells were differentiated for 6 to 72 h in EMEM SFM with GT1b, N2 and B27. Cells were treated with BoNT/A from 0.03 to 25 pM for 24 h followed by media change and 48 h incubation. The ECL-ELISA demonstrated that SiMa cells' sensitivity improved with ≥48 h differentiation. C. Optimization of BoNT/A treatment conditions. Differentiated SiMa cells were treated with 0.1 to 25 pM BoNT/A for 6 h or 24 h followed by incubation in toxin-free medium for 0, 16, 24, 48, or 72 h. Lysates were analyzed in the ECL-sandwich ELISA. EC50 values were similar under all treatment conditions tested but S/B values were different. Optimal BoNT/A treatment to generate a low EC50 and high S/B was 24 h followed by 2 or 3 days incubation. D. Optimization of the ECL-sandwich ELISA conditions. Concentrations of capture (2E2A6) and detection (S9684) antibodies were optimized. Capture antibody at 20 and 40 µg/mL was tested in combination with detection antibody at 1 and 5 µg/mL to analyze lysates from cells treated with BoNT/A from 0.01 to 25 pM. 2E2A6 at 20 µg/mL spotted in 5 µL combined with S9684 at 5 µg/mL in 25 µL was optimal (Error bars = std. dev.). E. Cell lysate incubation time and temperature were evaluated and optimal condition was 16 h incubation at 4°C.