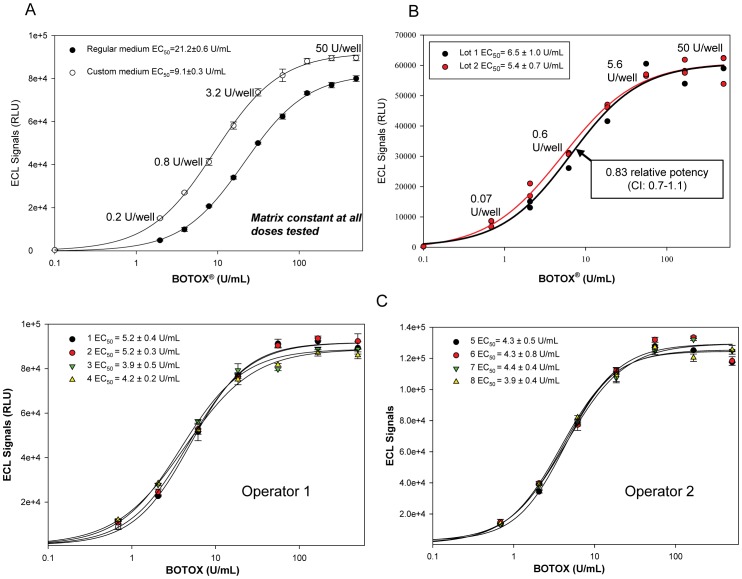
Figure 8. The CBPA can measure BoNT/A biological activity in BOTOX® vials.
A. A custom medium to reconstitute BOTOX® vials (the nominal value of 100 U was used) was designed to overcome the hypertonicity caused by NaCl present in the formulation. The matrix for the subsequent dilutions was kept constant. Performance of the assay was improved resulting in better sensitivity (EC50 = 0.9 U/well) and higher efficacy of uptake. B. Two different lots of BOTOX® (the nominal value of 100 U was used) were evaluated in the CBPA. Data was analyzed in PLA 2.0 resulting in 0.82 relative potency (CI: 0.7–1.1) indicating similar potency of both lots. C. A single lot of BOTOX® was tested by two operators (n = 8 and n = 9 independent experiments respectively), four runs for each operator are shown. Relative potencies were similar for all the tests performed demonstrating consistent performance by two operators.