Figure 3. Structural diagram which quantitatively assesses the effect of the addition or elimination of different structural elements from the flavonoid core on the ACEI activity of luteolin at 100 µM.
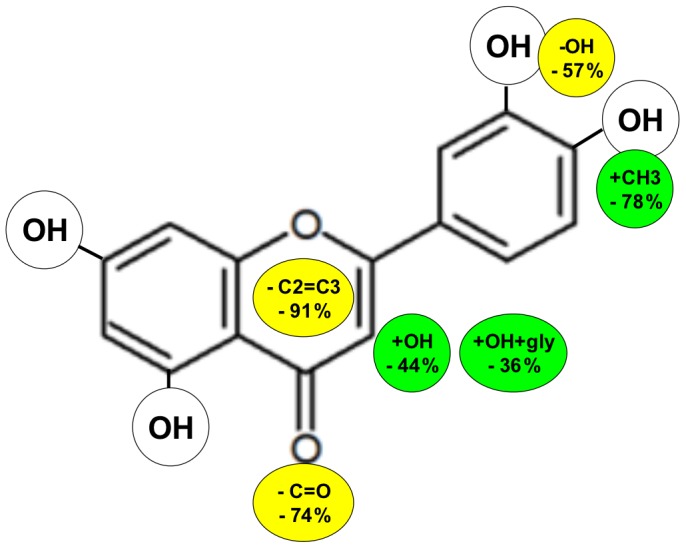
According to these data, the significance order was: double bond C2 = C3 (absence: −91% activity by comparing naringenin vs. apigenin) >4′-O-methoxylation (presence: −78% by comparing diosmetin vs. luteolin) ≈ 4-carbonyl group (absence: −74% by comparing epicatechin vs. luteolin) >3′-hydroxylation (absence: −57% by comparing apigenin vs. luteolin) >3-hydroxylation (presence: −44% by comparing quercetin vs. luteolin) >3-O-glycosylation (presence: -36% by comparing rutin vs. luteolin).
