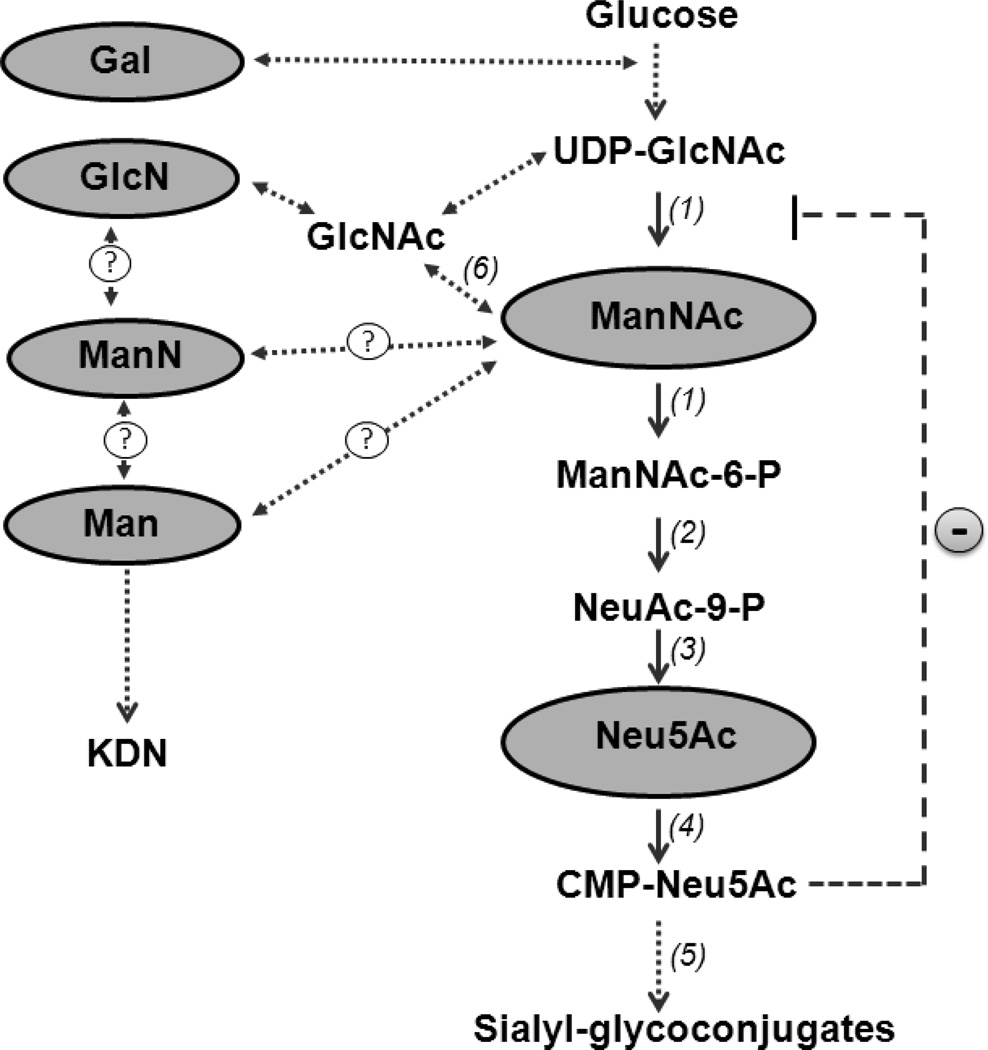
Fig. 1.
Sialic acid biosynthesis pathway. The synthesis of sialic acid is initiated in the cytosol, where glucose undergoes several modifications to eventually become sialic acid (Neu5Ac). The reactions catalyzed by the bifunctional enzyme UDP-GlcNAc 2-epimerase/ManNAc kinase (GNE/MNK) (1) are the initial and rate-limiting steps in this pathway. Subsequent steps are mediated by cytosolic NeuAc-9P synthetase (2) and NeuAc-9P phosphatase (3) to produce Neu5Ac, after which nuclear by CMP-Neu5Ac synthethase (4) produces CMP-Neu5Ac. Cytosolic CMP-Neu5Ac strongly feedback inhibits GNE-epimerase activity (−) and is utilized in the Golgi-complex by sialyltransferases (5) to produce sialyl-glycoconjugates. Monosaccharides that were orally administered to Gne p.M712T mice to test their sialylation-increasing potential are highlighted in gray. Neu5Ac and its precursor ManNAc are likely to increase sialylation by increasing the flux through the sialic acid synthesis pathway, since conversion of both these intermediates are unaffected by the feedback inhibition of the GNE-epimerase by CMP-Neu5Ac. GlcN may be converted into GlcNAc and then into ManNAc by GlcNAc 2-epimerase (6) or could increase UDP-GlcNAc pools and possibly increase flux into the sialic acid pathway. Gal, Man, or ManN may have potential, yet unknown (?), ways to enter the Neu5Ac biosynthesis pathway at one or more relevant steps, and may as such contribute to increased sialylation. Alternatively, Man or ManN may increase sialylation through production of an alternative sialic acid, KDN(2-keto-3-deoxy-D-glycero-D-galacto-nononic acid),which can be formed independent of the GNE/MNK-mediated Neu5Ac synthesis pathway.