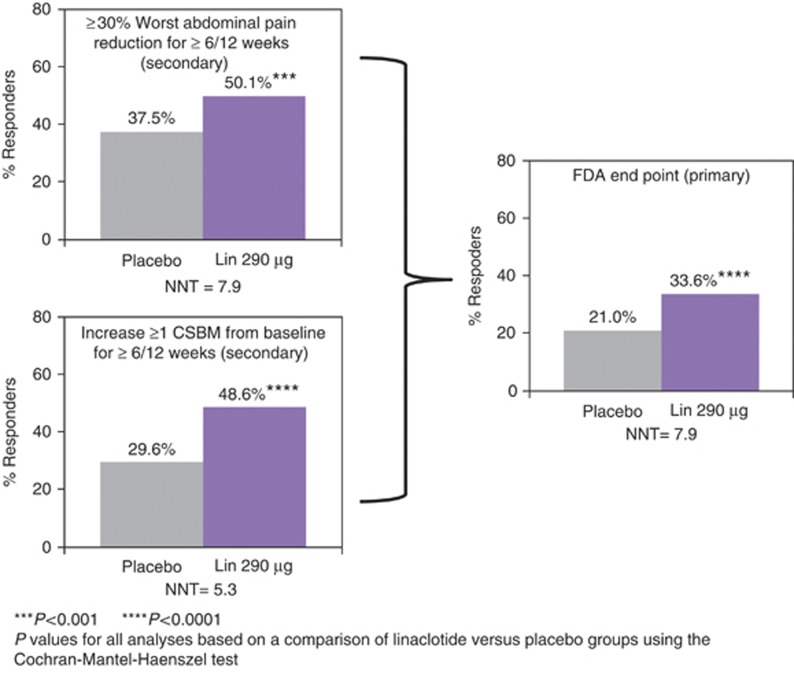
Figure 2.
FDA end point and components. FDA end point: ≥30% abdominal pain reduction and increase ≥1 CSBM from baseline in the same week for ≥6/12 weeks. ****P value <0.0001, ***<0.001 for linaclotide vs. placebo (Cochran–Mantel–Haenszel (CMH) test). P values met the criterion for statistical significance based on the multiple-comparison procedure. CSBM, complete spontaneous bowel movement; FDA, Food and Drug Administration; Lin, linaclotide; NNT, number needed to treat.