Figure 1. Identification of the Sample of Novel Therapeutic Applications for the Current Analysis.
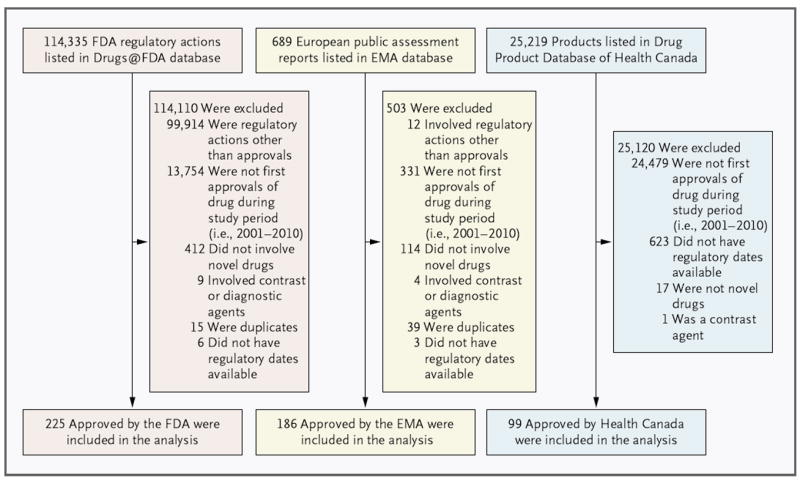
The analysis includes applications approved by the Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Health Canada from 2001 through 2010. Health Canada has posted Summary Basis of Decision documents, which list the dates needed to calculate review time, only for therapeutics approved since 2005. As a result, 623 applications were excluded from the analysis because regulatory dates were not available from Summary Basis of Decision documents.
