Figure 2. Median First-Review Times and Total Review Times for Applications for Novel Therapeutics Approved by the FDA, the EMA, and Health Canada.
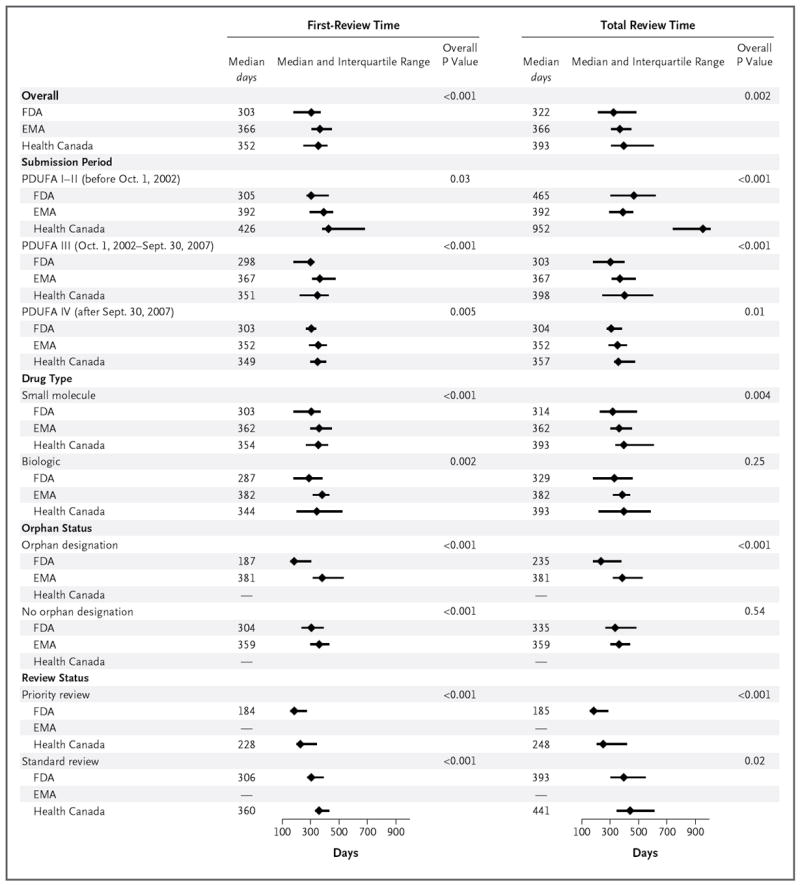
Information on application approvals by Health Canada is available only from 2005 on. Status with respect to priority or standard review was not analyzed for the EMA because the EMA Accelerated Assessment procedure, which is similar to priority review at the FDA, was not established until 2007. The overall P value is for the comparison across the three agencies. P<0.02 for all of the following comparisons: overall first-review time between the FDA and the EMA and between the FDA and Health Canada and overall total review time between the FDA and Health Canada; total review time during PDUFA I–II between the FDA and Health Canada and between the EMA and Health Canada; first-review and total review times during PDUFA III between the FDA and the EMA and between the FDA and Health Canada; first-review time during PDUFA IV between the FDA and the EMA and between the FDA and Health Canada and total review time during PDUFA IV between the FDA and Health Canada; and first-review time for small-molecule drugs between the FDA and the EMA and between the FDA and Health Canada, total review time for small-molecule drugs between the FDA and Health Canada, and first-review time for biologic agents between the FDA and the EMA.
