Abstract
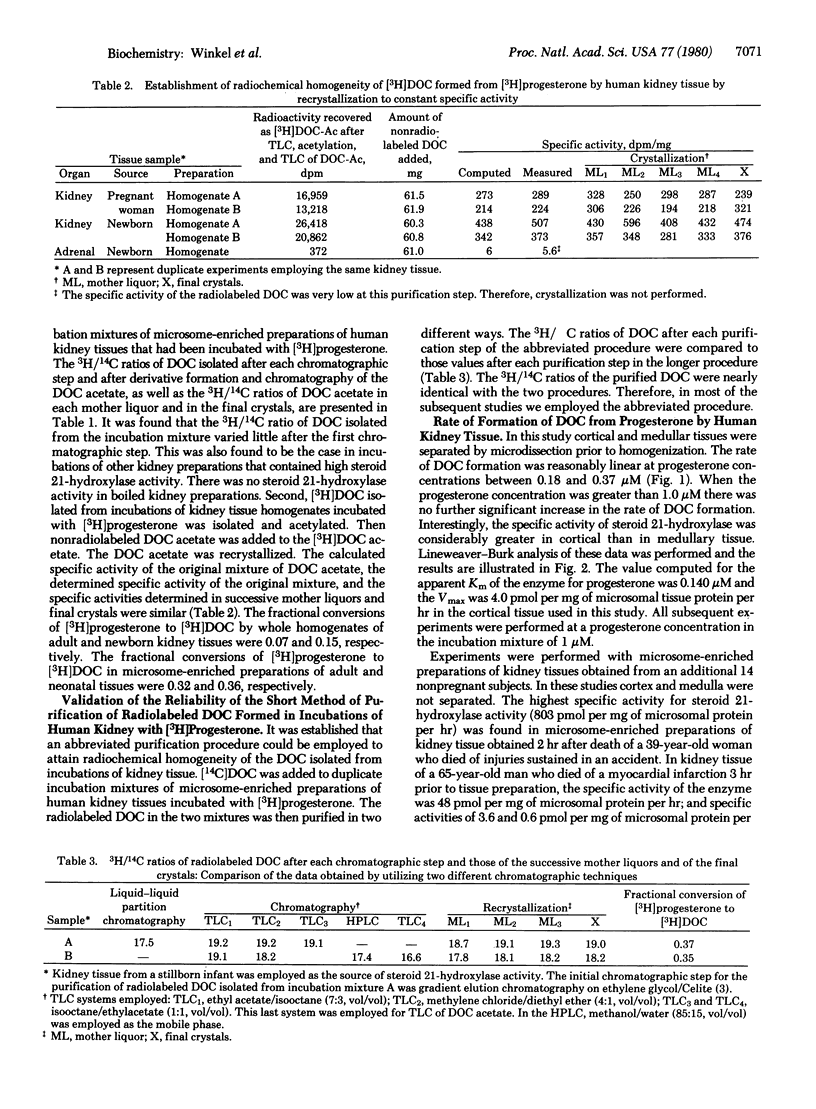
The extra-adrenal formation of deoxycorticosterone (DOC) from plasma progesterone has been demonstrated in humans. In those studies it was shown that in some persons the volume of plasma cleared of progesterone by DOC formation was great, namely, 75 liter/24 hr. Because steroid 231-hydroxylase activity [steroid 21-monooxygenase; steroid, hydrogen-donor:oxygen oxidoreductase (21-hydroxylating), EC 1.14.99.10] could not be demonstrated in homogenates or microsome-enriched preparations of human lung or liver tissue, we speculated that the 21-hydroxylation of plasma progesterone might take place in the kidney. Employing whole tissue homogenates and microsome-enriched preparations of human kidney tissue, we demonstrated the formation of [3H]DOC from kidney tissue, we demonstrated the formation of [3H]DOC from [3H]progesterone. The rate of formation of DOC from progesterone in microsomal preparations from kidney tissues of adult humans varied from 0 to 803 pmol per mg of microsomal protein per hr. The value computed for the apparent Km of the enzyme for progesterone was 0.140 microM. On the basis of these findings, we conclude that steroid 21-hydroxylase activity is present in human kidney tissue and that the kidney may be an important site of DOC formation as well as a site of DOC action.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACEVEDO H. F., AXELROD L. R., ISHIKAWA E., TAKAKI F. STUDIES IN FETAL METABOLISM. II. METABOLISM OF PROGESTERONE-4-C14 AND PREGNENOLONE-7ALPHA-H3 IN HUMAN FETAL TESTES. J Clin Endocrinol Metab. 1963 Sep;23:885–890. doi: 10.1210/jcem-23-9-885. [DOI] [PubMed] [Google Scholar]
- ARCOS M., GURPIDE E., WIELE R. L., LIEBERMAN S. PRECURSORS OF URINARY PREGNANEDIOL AND THEIR INFLUENCE ON THE DETERMINATION OF THE SECRETORY RATE OF PROGESTERONE. J Clin Endocrinol Metab. 1964 Mar;24:237–245. doi: 10.1210/jcem-24-3-237. [DOI] [PubMed] [Google Scholar]
- BOLTE E., MANCUSO S., ERIKSSON G., WIQVIST N., DICFALUSY E. STUDIES ON THE AROMATISATION OF NEUTRAL STEROIDS IN PREGNANT WOMEN. 3. OVER-ALL AROMATISATION OF DEHYDROEPIANDROSTERONE SULPHATE CIRCULATING IN THE FOETAL AND MATERNAL COMPARTMENTS. Acta Endocrinol (Copenh) 1964 Apr;45:576–599. doi: 10.1530/acta.0.0450576. [DOI] [PubMed] [Google Scholar]
- Baird D. T., Horton R., Longcope C., Tait J. F. Steroid dynamics under steady-state conditions. Recent Prog Horm Res. 1969;25:611–664. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez C., Milewich L., Holland O. B. Radioiodinated derivatives for steroid radioimmunoassay. Application to the radioimmunoassay of cortisol. J Lab Clin Med. 1977 Apr;89(4):902–909. [PubMed] [Google Scholar]
- Horton R., Tait J. F. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966 Mar;45(3):301–313. doi: 10.1172/JCI105344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDonald P. C., Rombaut R. P., Siiteri P. K. Plasma precursors of estrogen. I. Extent of conversion of plasma delta-4-androstenedione to estrone in normal males and nonpregnant normal, castrate and adrenalectomized females. J Clin Endocrinol Metab. 1967 Aug;27(8):1103–1111. doi: 10.1210/jcem-27-8-1103. [DOI] [PubMed] [Google Scholar]
- Milewich L., Gomez-Sanchez C., Madden J. D., MacDonald P. C. Isolation and characterization of 5alpha-pregnane-3,20-dione and progesterone in pepipheral blood of pregnant women. measurement throughout pregnancy. Gynecol Invest. 1975;6(5):291–306. [PubMed] [Google Scholar]
- Schöneshöfer M., Wagner G. G. Sex differences in corticosteroids in man. J Clin Endocrinol Metab. 1977 Oct;45(4):814–817. doi: 10.1210/jcem-45-4-814. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., MacDonald P. C. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966 Jul;26(7):751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Milewich L., Parker C. R., Jr, Gant N. F., Simpson E. R., MacDonald P. C. Conversion of plasma progesterone to deoxycorticosterone in men, nonpregnant and pregnant women, and adrenalectomized subjects. J Clin Invest. 1980 Oct;66(4):803–812. doi: 10.1172/JCI109918. [DOI] [PMC free article] [PubMed] [Google Scholar]


