Abstract
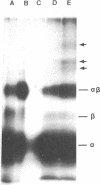
Lutropin, a pituitary hormone, and human choriogonadotropin bind to the same receptors in the ovary and elicit identical responses. A photoactivable derivative of human choriogonadotropin was used to identify the lutropin receptor on porcine granulosa cells. The hormone was condensed with a heterobifunctional reagent, the N-hydroxysuccinimide ester of 4-azidobenzoylglycylglycine, and iodinated. The 125I-labeled hormone (125I-hormone) derivative associated with the same number of receptors as 125I-hormone itself did but with a slightly lower Ka, 2.98 X 10(9) M-1 compared with 5.1 X 10(9) M-1 for 125I-hormone. The binding could be blocked with untreated hormone or lutropin but not with follitropin, prolactin, insulin, or bovine serum albumin. Its alpha and beta subunits could be crosslinked to produce alpha beta dimer by photolysis, the extent of crosslinking being dependent upon the reagent concentration used for the derivatization: 22.8% at 50 microM, 37.3% at 100 microM, and 67.2% at 150 microM. When the 125I-hormone derivative bound to the cells was photolyzed for crosslinking and the products resolved by electrophoresis on sodium dodecyl sulfate/polyacrylamide gels under reducing conditions, three new bands of lower electrophoretic mobility appeared in addition to alpha, beta, and alpha beta bands. Formation of these crosslinked complexes required photolysis and the presence of both cells bearing the receptor and the 125I-hormone derivative. It could be blocked by excess untreated hormone. The three bands correspond to molecular weights, 96,000 +/- 6,700, 66,000 +/- 4,600, and 63,000 +/- 4,400. Because the hormone has a high carbohydrate content and such glycoproteins are known to exhibit anomalous electrophoretic mobilities, these estimates must be tentative.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bregman M. D., Levy D. Labeling of glucagon binding components in hepatocyte plasma membranes. Biochem Biophys Res Commun. 1977 Sep 23;78(2):584–590. doi: 10.1016/0006-291x(77)90219-4. [DOI] [PubMed] [Google Scholar]
- Burleigh B. D., Liu W. K., Ward D. N. Photocoupling of the subunits of ovine lutropin using a specific aryl azide derivative of the beta subunit. J Biol Chem. 1978 Oct 25;253(20):7179–7185. [PubMed] [Google Scholar]
- Carney D. H., Glenn K. C., Cunningham D. D., Das M., Fox C. F., Fenton J. W., 2nd Photoaffinity labeling of a single receptor for alpha-thrombin on mouse embryo cells. J Biol Chem. 1979 Jul 25;254(14):6244–6247. [PubMed] [Google Scholar]
- Channing C. P., Kammerman S. Binding of gonadotropins to ovarian cells. Biol Reprod. 1974 Mar;10(2):179–198. doi: 10.1095/biolreprod10.2.179. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. H., Richards F. M. Reaction of a lipid-soluble, unsymmetrical, cleavable, cross-linking reagent with muscle aldolase and erythrocyte membrane proteins. J Biol Chem. 1977 Aug 10;252(15):5514–5521. [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. A novel approach to the identification of surface receptors. The use of photosensitive hetero-bifunctional cross-linking reagent. J Biol Chem. 1977 Mar 10;252(5):1566–1570. [PubMed] [Google Scholar]
- Ji T. H., Kiehm D. J., Middaugh C. R. Presence of spectrin tetramer on the erythrocyte membrane. J Biol Chem. 1980 Apr 10;255(7):2990–2993. [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan F. J., Birken S., Canfield R. E. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975 Jul 10;250(13):5247–5258. [PubMed] [Google Scholar]
- Siris E. S., Nisula B. C., Catt K. J., Horner K., Birken S., Canfield R. E., Ross G. T. New evidence for intrinsic follicle-stimulating hormone-like activity in human chorionic gonadotropin and luteinizing hormone. Endocrinology. 1978 May;102(5):1356–1361. doi: 10.1210/endo-102-5-1356. [DOI] [PubMed] [Google Scholar]
- Stouffer R. L., Tyrey L., Schomberg D. W. Changes in (125I) labeled human chorionic gonadotropin (hCG) binding to porcine granulosa cells during follicle development and cell culture. Endocrinology. 1976 Aug;99(2):516–525. doi: 10.1210/endo-99-2-516. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Muchmore D., Raftery M. A. Affinity-directed cross-linking of membrane-bound acetylcholine receptor polypeptides with photolabile alpha-bungarotoxin derivatives. Biochemistry. 1979 Nov 27;18(24):5511–5518. doi: 10.1021/bi00591a039. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Yeung C. W., Moule M. L. Photoaffinity labeling of insulin receptor of rat adiopocyte plasma membrane. J Biol Chem. 1978 Mar 25;253(6):1743–1745. [PubMed] [Google Scholar]