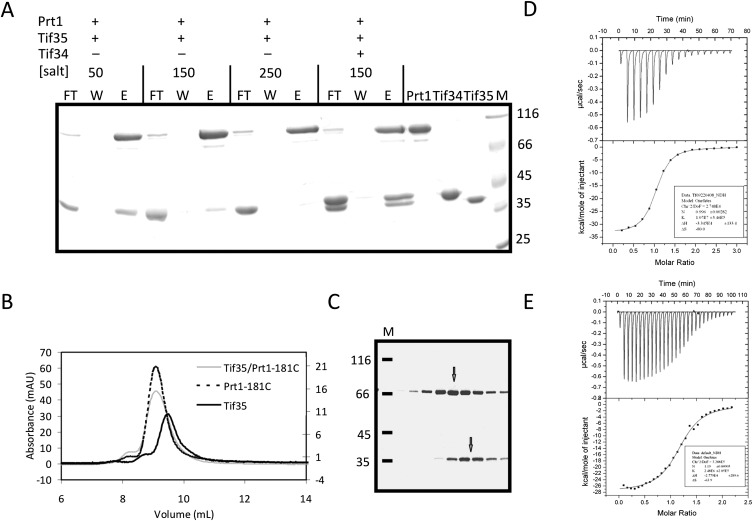
FIGURE 5.
Prt1 and Tif35 interact weakly in vitro. (A) Pull-down interaction studies between Tif35, full-length Prt1, and Tif34. Lanes FT, W, and E refer to the flow-through of the Ni-NTA beads, their last wash step, and the elution of the protein. (B) Overlay of the analytical SEC profiles of Prt1181C (dashed black), Tif35 (solid black), and Prt1181C-Tif35 mixture (solid gray) shows a broadening of the peak in the case of the mixture without any considerable shift the retention volume compared with individual components. (C) SDS-PAGE analysis of the peak of the mixture of Prt1181C-Tif35 shows that it is in fact composed of two adjacent peaks (marked by two arrows) as would be judged by the broadening of the peak. (D) The equimolar interaction of Tif35 and Tif34 is driven by the release of heat, which compensates for the decrease in entropy. (E) Interaction of Tif34 with Prt1181C is also equimolar and enthalpy driven. In both cases, the upper panels show raw data of heat effect (in μcal/sec) of injections of Tif34 into 1.5 mL of Tif35 (D) or Prt1181C (E). The lower panels show the fitted binding isotherms. The data points were obtained by integration of heat signals plotted against the molar ratio of Tif34 to either of the interaction partners in the reaction cell. The solid lines represent calculated curves using the best fit parameters obtained by nonlinear least squares fitting.