The endoplasmic reticulum (ER) is a fundamental cellular organelle responsible for a variety of physiological processes, including the synthesis, folding and post-translational modification of the majority of membrane and secreted proteins, calcium storage, and lipid synthesis among other important functions. Besides, many pathological conditions are associated with the occurrence of uncontrolled ER stress, including diabetes, neurodegeneration, and cancer.1
ER stress triggers the activation of the unfolded protein response (UPR), an adaptive program governing the recovery of homeostasis.2 Conversely, prolonged or irreversible ER stress triggers apoptosis, in which the activation of the canonical mitochondrial cell death pathway has an essential role in determining cell fate decisions.1 The UPR is mediated in part by the expression of distinct transcription factors, including XBP1, ATF6 and ATF4. Expression of ATF4 can promote apoptosis by upregulating several proapoptotic components of the BCL-2 family, including the BH3-only protein. This leads to BAX and BAK activation at the mitochondria and to the release of cytochrome c. Thus, the BCL-2 family of proteins has a pivotal role in the cross-talk between the ER and mitochondria to transduce cell death signals.
Many studies have shown that changes in the dynamics of the mitochondrial network (i.e., fission and fusion) contribute to cell death.3 In addition to alterations in the morphology of the mitochondria, fragmentation of the ER and the Golgi apparatus has been extensively described in dying cells. The ER is arranged in a highly complex three-dimensional network with distinct subdomains and diverse interconverting shapes, including tubules, sheet-like structures and lamellae, in addition to specialized contact sites with multiple organelles.4 Although it is becoming clear that many stress conditions induce drastic ER-morphological changes, the contribution of this process to apoptosis remains largely elusive. In this issue of Cell Death and Differentiation, Gerald Cohen and co-workers5 describe the existence of a rapid and reversible reorganization of the ER network that may represent an anticipated stress response. The main findings were obtained after treating cells with the pan-BCL-2 family antagonist apogossypol, which leads to a drastic and specific ER membrane remodeling (EMR), characterized by the clustering of the ER membrane into large and compact aggregates.5 This phenomenon was observed in a variety of mammalian cell lines and in the yeast Schizosaccharomyces pombe, suggesting the occurrence of an evolutionary conserved process. Interestingly, EMR correlated with a negative effect on protein trafficking through the secretory pathway, specifically affecting anterograde transport, in addition to decreasing the rate of protein synthesis. Moreover, inhibition of ER−Golgi vesicular trafficking also induced ER remodeling, indicating that the EMR is a dynamic stress response occurring in physiological conditions.5
Unexpectedly, despite the clear functional consequences of EMR upon BCL-2 family inhibition, this process was independent of the UPR. In fact, the authors provided strong genetic evidence indicating that none of the classical ER stress-signaling pathways, namely PERK/eIF2α, IRE1α/XBP1 and ATF6, were involved in EMR. In addition, BCL-2 inhibitors induced mild ER stress only after prolonged exposure (>8 h) as described previously (some examples in Albershardt et al.6 and Risberg et al.7), which dissociated from the fast kinetics of activation of EMR (<1 h).5 These findings were confirmed by global gene expression analysis, in which the signature observed after EMR did not match the changes triggered by classic ER stress-inducing agents. Interestingly, the mRNA signature of apogossypol treatment matched the profiles of cells treated with diverse compounds. Using this connectivity-mapping approach, the authors identified 20 novel structurally unrelated compounds that trigger EMR. Interestingly, most identified compounds shared the ability to perturb calcium homeostasis. Accordingly, inhibitors of the SERCA pump, such as thapsigargin, triggered EMR.
As apogossypol inhibits several anti-apoptotic BCL-2 family members, the authors performed knockdown experiments to target major anti-apoptotic proteins, including MCL-1, BCL-2, BCL-W and BCL-XL. Surprisingly, only silencing of MCL-1 triggered EMR. In addition, other BCL-2 family inhibitors such as TW-37 and ABT-737 (which only target BCL-2/BCL-XL, and not MCL-1) induced EMR, suggesting that more members of the family may be part of the pathway.5 These results suggest that MCL-1 may have a novel function beyond apoptosis, controlling ER morphogenesis or dynamics. However, the mechanism involved in the process and the possible physiological relevance were not addressed in the study. We speculate that depending on the intensity of the stress stimuli, EMR may serve as an adaptive reaction to cope with stress (i.e., an event to eliminate/isolate damaged ER), or may even contribute to cell death due to chronic ER injury.
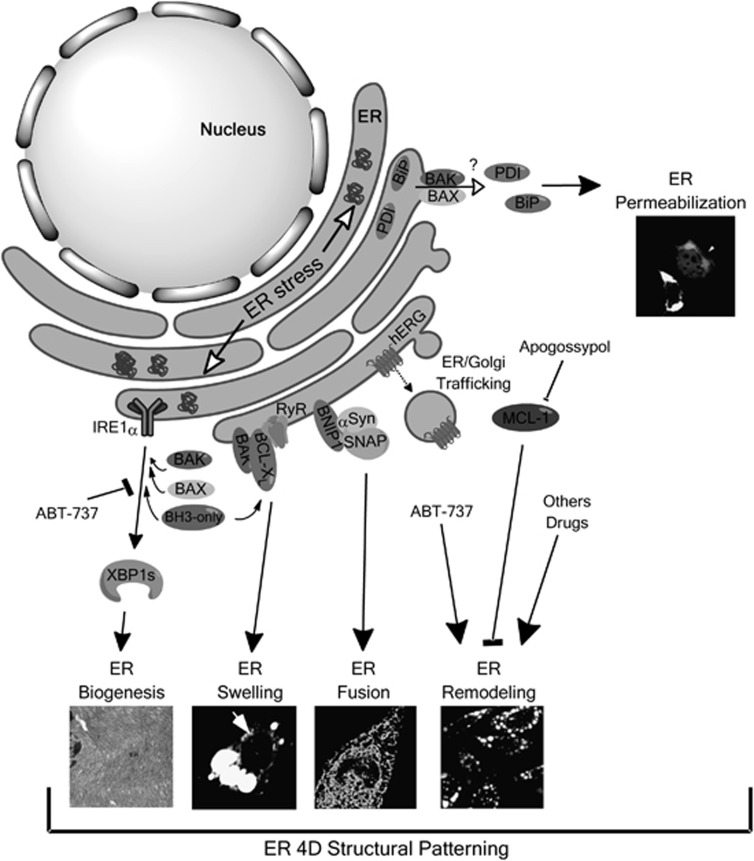
Of note, several studies support the role of the BCL-2 family in defining ER structure and function (Figure 1). So far, the best-characterized activity of this protein family at the ER membrane is the regulation of ER calcium homeostasis (reviewed in Hetz and Glimcher12). In addition, BCL-XL was shown to induce a drastic swelling of the ER lumen through the specific interaction with BAK and not with BAX.8 BH3-only proteins were also shown to modulate ER swelling. Other components of the BCL-2 family can modulate ER dynamics. For example, the BH3-only protein, BNIP1, controls ER tubule fusion by binding to syntaxin-18, an ER-located SNARE involved in membrane trafficking between the ER and Golgi.9 In addition, a network of BCL-2 family members, including BAX, BAK, BCL-2 and several BH3-only proteins, have an alternative function at the ER membrane, where they control UPR signaling, possibly because of a direct binding to the stress sensor IRE1α.13, 14 This pathway can enhance ER expansion (organelle biogenesis) through the expression of the transcription factor XBP113 and the downstream upregulation of phospholipid synthesis. Finally, initial studies addressing the function of BAX and BAK at the ER membrane revealed that ER stress triggers the specific oligomerization of these proteins in this compartment.15 A recent report suggested that BAX and BAK activation at the ER triggers ER permeabilization, leading to the release of ER proteins, including the foldase PDI.10 Importantly, PDI was recently shown to have a pro-apoptotic activity through its translocation to ER−mitochondria contact sites.16 BAX/BAK-dependent ER permeabilization is antagonized by BCL-XL and enhanced by BH3-only proteins.10 Together, these studies suggest a relevant function of the BCL-2 family in integrating information to shape the three-dimensional structure and dynamics of the ER.
Figure 1.
Modulation of ER structure and dynamic by the BCL-2 family of proteins. Schematic representation of the known functions of BCL-2 family members at the ER in terms of stimulating ER biogenesis through the UPR, ER lumen swelling, ER membrane fusion, remodeling and permeabilization. Examples are also shown to illustrate the morphological changes associated with these novel activities of BCL-2 family members. Copyright authorization were obtained for references5, 8–11. In brief, ER biogenesis: Electron microscopy of the ER network in pancreatic−acinar cells. XBP1 deficiency ablates ER biogenesis in vivo. ER swelling: HEK cells were cotransfected with expression vectors for BAK and BCL-XL. Then ER swelling was monitored by the expression of ER-targeted RFP. ER fusion: Silencing of BNIP1 in Hela cells induces fission of ER tubules. The ER network was visualized after the expression of GFP-cytochrome b5 fusion protein. ER membrane remodeling: Inhibition of MCL-1 with apogossypol induces the remodeling of the ER membrane into reversible clusters as visualized after staining of the integral ER protein BAP31. ER permeabilization: MEF cells expressing an ER-tagged and -soluble version of YFP were treated with an ER stress agent. Then the redistribution of YFP into the cytosol was observed on a Bak/Bax-dependent manner (white arrow)
As inhibition of anti-apoptotic BCL-2 family members induces EMR, an important question to address is whether or not the release of BH3-only proteins and/or the activation of BAX and BAK at the ER are required for this process. In addition, as EMR occurs in yeast, it may be interesting to test if the recently identified BH3-only protein, Ynl305cp,17 contributes to the process in this model organism. Interestingly, 6 years ago we described similar EMR events in cells treated with thapsigargin,13 which were interpreted at that time as changes in ER size and/or biogenesis. Interestingly, in that study we showed that EMR was fully blocked in BAX and BAK double-deficient cells, and was independent of the release of calcium to the cytosol,13 consistent with Cohen's studies.
Many interesting questions arise from this report. How does ER membrane rearrange into distinct clusters? Remarkably, a wealth of information has timely emerged from the field of ER morphogenesis, for which many components that shape the ER and generate specific subdomains have been recently identified.4, 18, 19 Thus, it will be interesting to define the molecular machinery underlying EMR by MCL-1. For example, is the cytoskeleton involved in EMR? This is an interesting possibility to explore, as a tight association between EMR and the microtubule cytoskeleton based on two well-described mechanisms, ER-tip attachment complexes and an ER-sliding, has been described.4 Along these lines, studies have already described a direct connection between BCL-2 and actin polymerization.20 In addition, it will be interesting to define whether signaling pathways that originate at MCL-1 impinge on the function of proteins that define the architecture of ER sheets, such as components of the traslocon family, or tubules, such as atlastins, reticulons and DP1/Yop1p.4, 18, 19 Finally, as the ER has close contacts with virtually every cellular organelle, it remains to be determined if EMR could affect the physical and functional interaction between the ER and other subcellular compartments.
Although the authors suggested that EMR may represent a novel stress pathway, additional studies are needed to define this possibility. So far the data presented in Cohen's study indicates that EMR is a rapid and reversible process induced by diverse stimuli, and the evidence suggesting that the functional consequences of EMR are only correlative. EMR may be an important feature in human pathologies. The authors noticed that many of the compounds identified as triggering EMR have been previously linked to cardiac abnormalities (known as long-QT syndrome). This condition is often associated with hERG pore blocking. In agreement with this, the authors showed that apogossypol also inhibited hERG channel activity, suggesting that EMR may be associated with human disease.
In conclusion, this study, together with previous findings, opens a novel and exciting scenario, in which the modulation of ER dynamics by BCL-2 family members may have important consequences to diverse physiological and pathological conditions. Based in the discovery of ER sub-compartments with specialized functions,4, 18, 19 the fine tuning of the ER network in terms of its three-dimensional assembly and responsiveness to stress conditions suggest the possibility that EMR may operate as a conserved homeostatic response to adjust ER function to a fluctuating environment.
Acknowledgments
We thank Andres Couve for his insightful comments. This work was supported by FONDAP, grant no. 15010006, Millennium Institute No. P09-015-F and ACT1109 (CH), and a CONICYT PhD fellowship (HU).
References
- Hetz C. Nat Rev Mol Cell Biol. 2012. pp. 89–102. [DOI] [PubMed]
- Walter P, Ron D. Science. 2011. pp. 1081–1086. [DOI] [PubMed]
- Martinou JC, Youle RJ. Dev Cell. 2011. pp. 92–101. [DOI] [PMC free article] [PubMed]
- Friedman JR, Voeltz GK. Trends Cell Biol. 2011. pp. 709–717. [DOI] [PMC free article] [PubMed]
- Varadarajan S, et al. Cell Death Differ. 2012. pp. 1896–1907. [DOI] [PMC free article] [PubMed]
- Albershardt TC, et al. J Biol Chem. 2011. pp. 24882–24895. [DOI] [PMC free article] [PubMed]
- Risberg K, Fodstad O, Andersson Y. PLoS One. 2011. p. e24012. [DOI] [PMC free article] [PubMed]
- Klee M, Pimentel-Muinos FX. J Cell Biol. 2005. pp. 723–734. [DOI] [PMC free article] [PubMed]
- Nakajima K, et al. EMBO J. 2004. pp. 3216–3226. [DOI] [PMC free article] [PubMed]
- Wang X, et al. Cell Death Differ. 2011. pp. 38–47. [DOI] [PMC free article] [PubMed]
- Lee AH, et al. EMBO J. 2005. pp. 4368–4380. [DOI] [PMC free article] [PubMed]
- Hetz C, Glimcher L. Trends Cell Biol. 2008. pp. 38–44. [DOI] [PubMed]
- Hetz C, et al. Science 2006312572–576.16645094
- Rodriguez DA, et al. EMBO J. 2012. pp. 2322–2335. [DOI] [PMC free article] [PubMed]
- Zong WX, et al. J Cell Biol. 2003. pp. 59–69. [DOI] [PMC free article] [PubMed]
- Hoffstrom BG, et al. Nat Chem Biol. 2010. pp. 900–906. [DOI] [PMC free article] [PubMed]
- Buttner S, et al. EMBO J. 2011. pp. 2779–2792. [DOI] [PMC free article] [PubMed]
- Park SH, Blackstone C. EMBO Rep. 2010. pp. 515–521. [DOI] [PMC free article] [PubMed]
- Ramirez OA, Couve A. Trends Cell Biol. 2011. pp. 219–227. [DOI] [PubMed]
- Ke H, et al. Cell Res. 2010. pp. 458–469. [DOI] [PMC free article] [PubMed]