Abstract
T cells developing in the thymus undergo rigorous positive and negative selection to ensure that those exported to peripheral lymphoid organs bear T-cell receptors (TCRs) capable of reacting with foreign antigens but tolerant of self. At each checkpoint, whether a thymocyte survives or dies is determined by antiapoptotic and proapoptotic Bcl-2 family members. We used Mcl-1 transgenic (tg) mice to investigate the impact of elevated expression of antiapoptotic Mcl-1 on thymocyte apoptosis and selection, making a side-by-side comparison with thymocytes from BCL-2tg mice. Mcl-1 was as effective as Bcl-2 at protecting thymocytes against spontaneous cell death, diverse cytotoxic insults and TCR–CD3 stimulation-driven apoptosis. In three different TCR tg models, Mcl-1 markedly enhanced positive selection of thymocytes, as did Bcl-2. In H-Y TCR tg mice, elevated Mcl-1 and Bcl-2 were equally effective at inhibiting deletion of autoreactive thymocytes. However, in the OT-1tg model where deletion is mediated by a peripheral antigen whose expression is regulated by Aire, Mcl-1 was less effective than Bcl-2. Thus, the capacity of Mcl-1 overexpression to inhibit apoptosis triggered by TCR stimulation apparently depends on the thymocyte subset subject to deletion, presumably due to differences in the profiles of proapoptotic Bcl-2 family members mediating the deletion.
Keywords: Mcl-1, Bcl-2, Bim, apoptosis, thymic selection, transgenic mice
T cells developing in the thymus are submitted to rigorous selection procedures.1 Early cortical thymic progenitors (known as double-negative (DN) thymocytes because they do not express either the CD4 or CD8 coreceptor) undergo rearrangement of the T-cell receptor (TCR)β gene locus and those achieving a functional rearrangement express a pre-TCR (composed of the TCRβ chain associated with the invariant pre-Tα chain). Signalling through this pre-TCR complex promotes survival and continued differentiation of the CD4/CD8 double-positive (DP) stage, whereupon TCRα gene rearrangement commences. Productive TCRα rearrangement enables expression of surface-bound αβTCR along with the attendant CD3 signalling complex. DP thymocytes with TCRs that fail to engage major histocompatibility complex (MHC)–peptide complexes (the majority) die ‘by neglect' within a few days. In contrast, those that bear TCRs capable of binding MHC–peptide complexes receive a survival and differentiation signal (‘positive selection') and migrate to the thymic medulla2 to mature into MHC class I-restricted CD8 single-positive (CD8SP) or MHC class II-restricted CD4SP thymocytes. Further selection then takes place, to prevent self-reactive T cells exiting the thymus to peripheral lymphoid organs. Thymocytes that express TCRs that bind MHC–peptide ligands with an inappropriately high avidity undergo ‘negative selection', primarily by induction of apoptosis, although other mechanisms such as anergy and receptor editing are thought to contribute.3 Notably, medullary epithelial cells expressing the transcriptional regulator Aire (autoimmune regulator) produce a broad range of peripheral tissue antigens for presentation to the maturing thymocytes and thereby extend the scope of negative selection.4
At each of the checkpoints, thymocyte life or death is primarily determined by the balance between opposing factions of the Bcl-2 protein family, which associate at the outer mitochondrial membrane to regulate ‘intrinsic' or stress-induced apoptosis.5 If the Bcl-2 pro-survival family members prevail, mitochondrial membrane integrity is preserved. If, however, proapoptotic relatives known as BH3-only proteins are induced, they bind tightly to the hydrophobic groove on the surface of the pro-survival proteins, preventing them from neutralising other proapoptotic relatives, Bax and Bak (which have multiple BH- (Bcl-2 homology) domains). As a consequence, Bax and Bak homo-oligomerize, provoking permeabilisation of the outer mitochondrial membrane, release of cytochrome c into the cytoplasm and activation of caspases, which trigger apoptosis by cleaving vital cellular proteins.
The prominence of individual pro-survival Bcl-2 family members varies during thymocyte development. Although Bcl-2 is higher in DN and SP cells than in DP thymocytes,6, 7 the inverse is true for Bcl-xL8 and perhaps also for A19 (see, however, Verschelde et al.10). Mcl-1 is expressed at comparable levels in all of these cell subsets.11 Bim is the BH3-only protein most critical for thymocyte deletion: thymocytes lacking Bim were refractory to apoptosis induced by stimulation with CD3 antibodies and Bim deficiency in transgenic (tg) mice expressing autoreactive TCRs severely impaired thymocyte apoptosis.12, 13
Studies using tg mice showed that overexpression of Bcl-213 or Bcl-xL8 enhanced the survival of thymocytes (and mature T cells) exposed to diverse cellular stresses. Self-reactive T cells did not accumulate in the peripheral lymphoid organs of these tg mice, but whether deletion of autoreactive thymocytes had been impaired was more controversial. One group,14, 15 but not another,16 concluded that deletion of thymocytes reactive to self-superantigens was impeded by overexpression of Bcl-2. In another model, overexpression of Bcl-2 was found to reduce the deletion of self-reactive thymocytes,12, 15 whereas overexpression of Bcl-xL did not.8
Mcl-1, the most divergent pro-survival protein, has a distinctive interaction profile with its proapoptotic relatives. Similar to all pro-survival Bcl-2 family members, it binds the BH3-only proteins Bim, tBid and Puma with high affinity. However, similar to A1, it also binds strongly to Noxa, but fails to bind to Bad, whereas the opposite holds for Bcl-2, Bcl-xL and Bcl-w.17, 18 Furthermore, Mcl-1 restrains activation of the multi-domain Bak protein but Bcl-2 does not.19, 20
Mcl-1 is essential for thymocyte survival at both the DN and SP stages but shows functional redundancy with Bcl-xL at the DP stage.11, 21 It was recently shown that Mcl-1-deficient thymocytes die largely due to a Bak-specific mechanism and cannot be rescued by Bcl-2 overexpression.22 This observation raised the possibility that overexpression of Mcl-1 might affect thymocyte survival and development differently than overexpression of Bcl-2.
To investigate this possibility, we have used the same tg vector to compare the impact of elevated Mcl-1 and BCL-2 protein on thymocyte apoptosis following exposure to cytotoxic agents and explored the consequences for positive and negative thymic selection in three different TCR tg mouse models.
Results
Elevated Mcl-1 protects thymocytes under diverse cytotoxic conditions
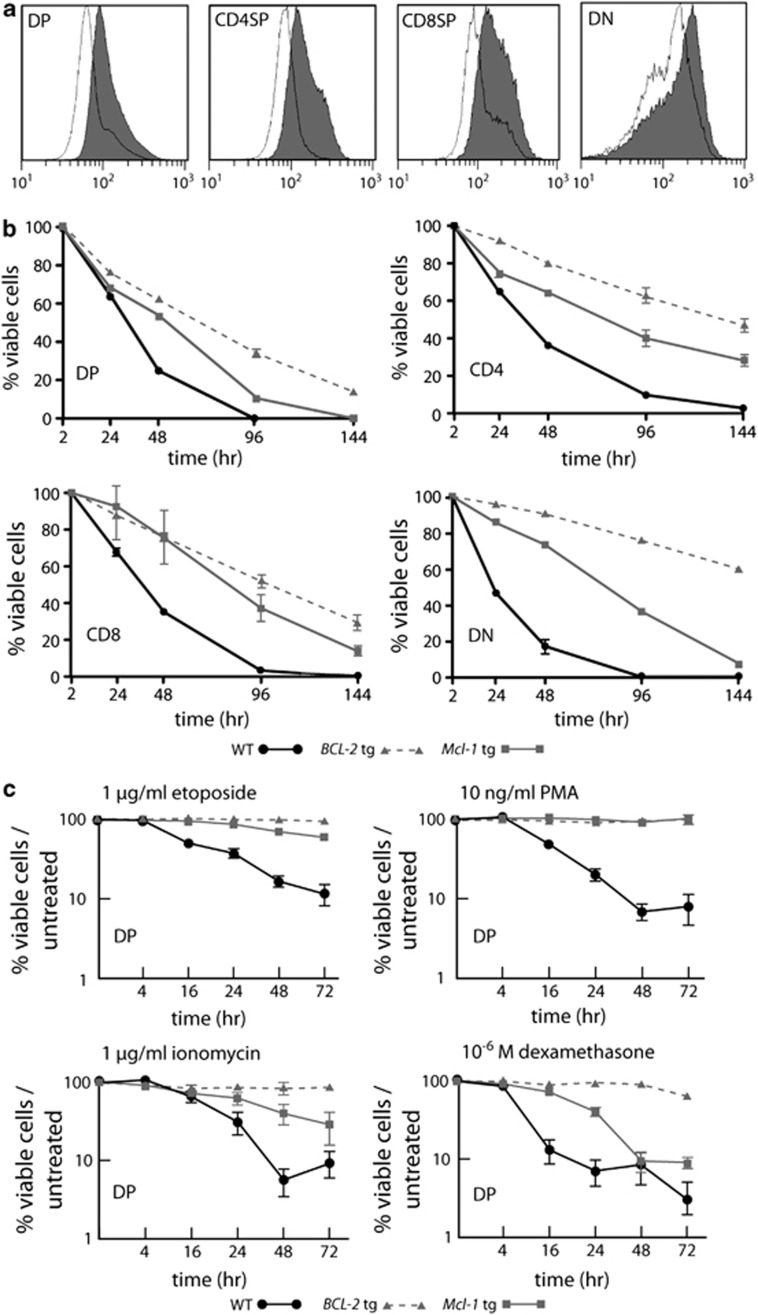
The FLAG-tagged tg mouse Mcl-1 protein was readily detected in DP, CD4SP, CD8SP and DN thymocytes from vavP-Mcl-1tg (hereafter Mcl-1tg) mice23 (Figure 1a) and each of these populations showed enhanced survival when cultured in vitro in medium lacking cytokines, as did those from vavP-BCL-2 tg (hereafter BCL-2tg) mice, which express high levels of human BCL-2 protein (Figure 1b). Overexpression of Mcl-1 increased the resistance of DP thymocytes, to γ-irradiation,23 etoposide, phorbol 12-myristate 13-acetate (PMA), ionomycin and dexamethasone (Figure 1c). Their resistance under these conditions was comparable to that of DP thymocytes from BCL-2tg mice, except for dexamethasone treatment where resistance was more durable for the BCL-2 overexpressing cells.
Figure 1.
Mcl-1 overexpression protects thymocytes against apoptosis in vitro. (a) Histograms showing tg Flag-tagged Mcl-1 protein expression in thymocyte subpopulations from Mcl-1tg (grey fill) or control WT (no fill) mice. Data representative of three independent experiments with one mouse of each genotype. (b) Mcl-1 overexpression enhances the survival of DP, CD4SP, CD8SP and DN thymocytes cultured in conventional medium lacking cytokines. WT (black line; n=3), Mcl-1tg (grey line; n=3), BCL-2tg (broken grey line, n=3). Points represent mean±S.E.M. (c) Mcl-1 overexpression protected DP thymocytes against apoptosis induced by 1 μg/ml etoposide, 10 ng/ml PMA, 1 μg/ml ionomycin or 1 μM dexamethasone. Cell viability was calculated relative to the viability of cells cultured in medium alone (see Figure 1b) to show stimulus-specific apoptosis. WT (black line, n=6-9), Mcl-1tg (grey line, n=5-9), BCL-2tg (broken grey line, n=2−4). Points represent mean (±S.E.M.). Under the conditions tested, the Mcl-1tg and BCL-2tg conferred statistically comparable protection against etoposide. PMA and ionomycin at each time point, with the exception of ionomycin treatment for 72 h, where the BCL-2tg was more protective (P≤0.05). In contrast, the Mcl-1tg was not as protective as the BCL-2tg against dexamethasone (t=24, 48, 72 h, P<0.001) Student's t-test. Thymocytes were labelled with fluorochrome-conjugated antibodies and sorted before culture. Viable cells (negative for PI and annexin V) were enumerated by flow cytometry at the times indicated
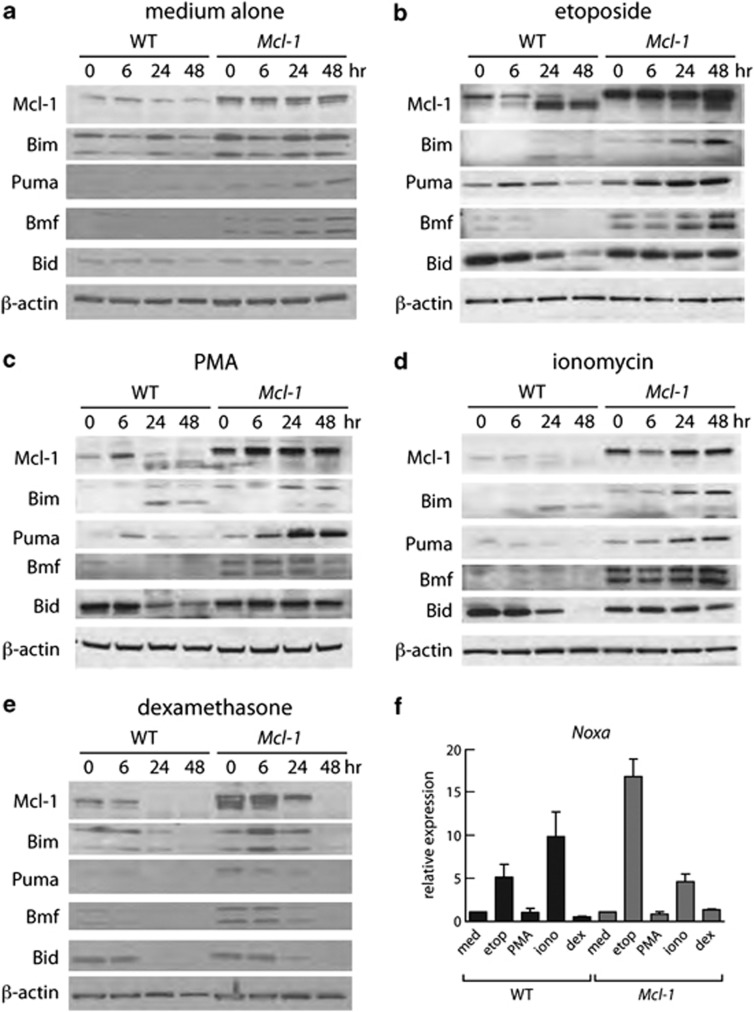
Western blot analysis (Figures 2a–e) showed that, before treatment, the levels of certain BH3-only proteins, notably Bim, Bmf and Puma, were higher in thymocytes from Mcl-1tg than wild-type (WT) mice, presumably because higher Mcl-1 levels preserve cells that would otherwise die due to elevated BH3-only proteins and/or because sequestration of these BH3-only proteins by Mcl-1 retards their degradation, as previously observed in cells overexpressing BCL-2.24, 25 Following exposure to cytotoxic agents, the level of BH3-only proteins increased in both WT and Mcl-1tg thymocytes, in a pattern consistent with previous findings.26 Thus, for the DNA-damaging agent etoposide, there was a marked increase in Puma, Bim and Noxa (Figures 2b and f); for PMA, an increase in Bim and Puma; for ionomycin, an increase in Bim and Puma proteins and Noxa RNA; and for dexamethasone, an increase in Bim (Figure 2e). For WT thymocytes, each of the cytotoxic agents provoked a major shift in the mobility of endogenous Mcl-1 by 24 h, presumably reflecting modification and/or degradation.27 In the Mcl-1tg thymocytes, however, the level of intact Mcl-1 stayed high over the course of treatment with most agents, presumably accounting for the improved viability despite the increase in BH3-only proteins. Exposure to dexamethasone, in contrast, resulted in a decrease in Mcl-1 within 24 h, explaining the greater sensitivity of the tg cells to this agent. When the Mcl-1tg thymocytes were treated with dexamethasone in the presence of the pan-caspase inhibitor QVD-OPH, however, Mcl-1 levels remained high for 48 h (Supplementary Figure 1), suggesting that degradation is a downstream consequence of caspase activation.
Figure 2.
Mcl-1 levels correlate with thymocyte viability. (a– e) Western blot analysis (20 μg protein) of lysates of thymocytes from WT or Mcl-1tg mice cultured in vitro for indicated times with (a) medium alone, (b) 1 μg/ml etoposide, (c) 10 ng/ml PMA, (d) 1 μg/ml ionomycin or (e) 1 μM dexamethasone. Data are representative of three independent experiments each performed with thymocytes from one mouse of each genotype. Supplementary Table S1 shows quantification of protein levels. (f) Q-PCR analysis of Noxa mRNA expression after culture for 6 h under the conditions described in (a– e). The data are expressed relative to β-actin and the untreated sample for the same genotype. Bim was the only other gene tested that showed significant transcriptional activation at 6 h, and only in Mcl-1tg thymocytes in response to dexamethasone (∼4-fold) (data not shown)
Mcl-1 overexpression increases resistance to CD3 antibody-induced apoptosis
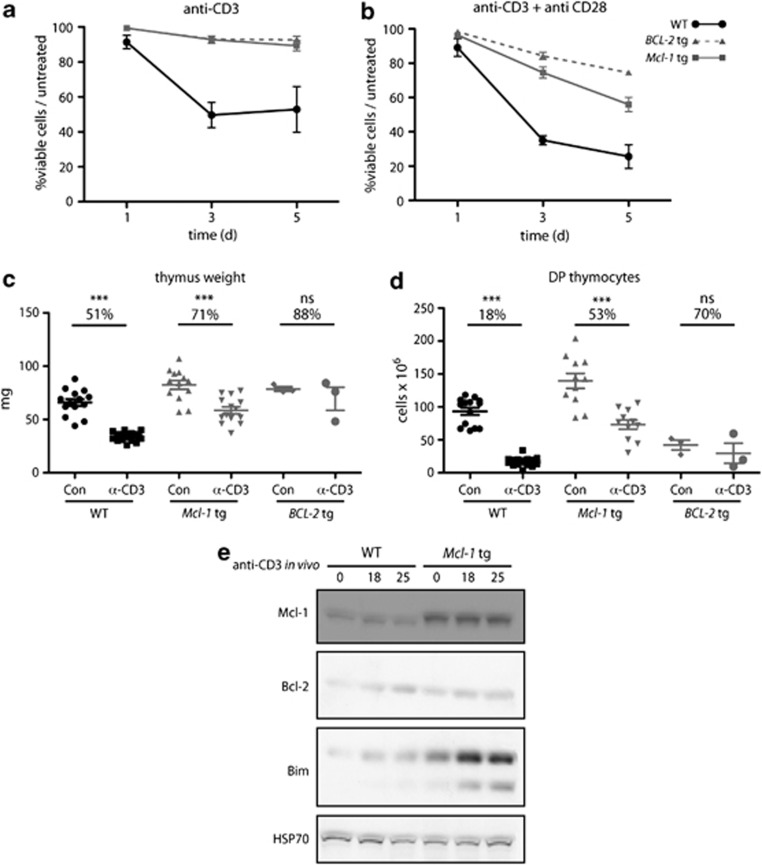
To determine whether Mcl-1tg thymocytes were resistant to TCR stimulation-mediated deletion, we first tested their sensitivity to apoptosis triggered by CD3 antibody-induced aggregation of the TCR–CD3 complex. In vitro stimulation of thymocytes with plate-bound antibodies to CD3 (or CD3 plus CD28) showed that DP thymocytes from Mcl-1tg mice survived significantly better than those from WT mice and that the resistance of the Mcl-1tg thymocytes was similar to that of BCL-2tg thymocytes (Figures 3a and b). The less-effective protection provided by the Mcl-1tg than the BCL-2tg to the stronger apoptotic signal delivered by anti-CD3 plus anti-CD28 seems likely to be due to the lower level of the tg protein achieved with the former (∼2–4-fold; see Materials and Methods).
Figure 3.
Overexpression of Mcl-1 protects thymocytes against CD3 antibody-induced apoptosis in vitro and in vivo. (a and b) Thymocytes from WT, Mcl-1tg and BCL-2tg mice were cultured in vitro in plates coated with (a) 10 μg/ml CD3 antibody or (b) 10 μg/ml CD3 antibody plus 10 μg/ml CD28 antibody and the percentages of viable DP thymocytes relative to untreated controls (thymocytes cultured in medium alone) were determined at the indicated time points as described in Materials and Methods. For each genotype, n=4 mice; points represent mean (±S.E.M.). Under the conditions tested, the Mcl-1tg and BCL-2tg conferred statistically comparable protection against anti-CD3 and anti-CD3+anti-CD28 in vitro at all time points, with the exception of anti-CD3+anti-CD28 treatment for 3 days and 5 days, where the BCL-2tg was more protective (P<0.05 and 0.01, respectively). (c– d) WT (n=11-14), Mcl-1tg (n=11-14) or BCL-2tg (n=3) mice were injected i.p. with 30 μg of TCRγ antibody (Con) or 30 μg CD3 antibody and (c) thymus weights and (d) numbers of DP thymocytes were determined after 40 h. Data points represent individual mice and bars represent mean±S.E.M. Percentage reduction relative to controls (control antibody injected animals) are indicated. ***P≤0.001; ns, not significant; Student's t-test. Similar results were also observed using vavP-Mcl-1(4) mice23 (data not shown), a line having lower levels of tg Mcl-1 expression. (e) Western blot analysis of thymocytes from WT and Mcl-1tg mice harvested at the indicated times following i.p. injection of 30 μg CD3 antibody. Each lane represents an individual mouse and data are representative of two independent experiments. Supplementary Table S2 shows quantification of protein levels
To more closely approximate physiological deletion of self-reactive thymocytes, we next determined the response to in vivo treatment with antibodies to CD3 (Figures 3c and d). As expected, injection of CD3 antibodies significantly depleted thymocytes in WT mice: after 40 h, thymus weight was reduced to 51% and DP thymocytes were depleted to 18% compared with controls. In contrast, α-CD3-injected Mcl-1tg mice retained 71% of thymus weight and 53% of DP thymocytes relative to controls. This rescue was comparable to that observed for BCL-2tg mice where 50–70% of DP thymocytes remained viable compared with controls (Figure 3 and Bouillet et al.12).
Bim is the major trigger of apoptosis induced by CD3 antibody12 and, accordingly, thymocytes from mice injected with α-CD3 showed increased Bim levels by 18 h (Figure 3e). As noted above, the basal level of Bim was higher in thymocytes from Mcl-1tg mice compared with those of WT mice and accumulated at much higher levels following TCR–CD3 signalling. Thus, the increased level of Mcl-1 achieved by transgene expression suffices to counteract the apoptosis normally induced by upregulation of Bim.
Overexpression of Mcl-1 alters the selection of autoreactive thymocytes
To explore whether the Mcl-1 transgene could confer protection against apoptosis induced by TCR overstimulation in more physiological settings, we turned to three well-established tg models of in vivo thymocyte deletion.
In the H-Y TCRαβ tg (hereafter H-Ytg) mouse, most thymocytes express a TCRαβ that recognises the male H-Y antigen presented by class I MHC H2-Db molecules.28 In female C57BL/6 H-Ytg mice, thymocytes expressing the tg TCR are positively selected to become mature CD8SP T cells that are exported to the periphery. In male mice, however, as soon as thymocytes express the H-Y TCR (during the DN to DP transition), they interact with MHC complexes presenting the ubiquitous H-Y antigen, causing a strong TCR signal that induces their deletion by Bim-mediated apoptosis.12 We crossed Mcl-1tg and BCL-2tg mice with H-Ytg mice and compared thymocyte populations in male and female progeny at 6–8 week.
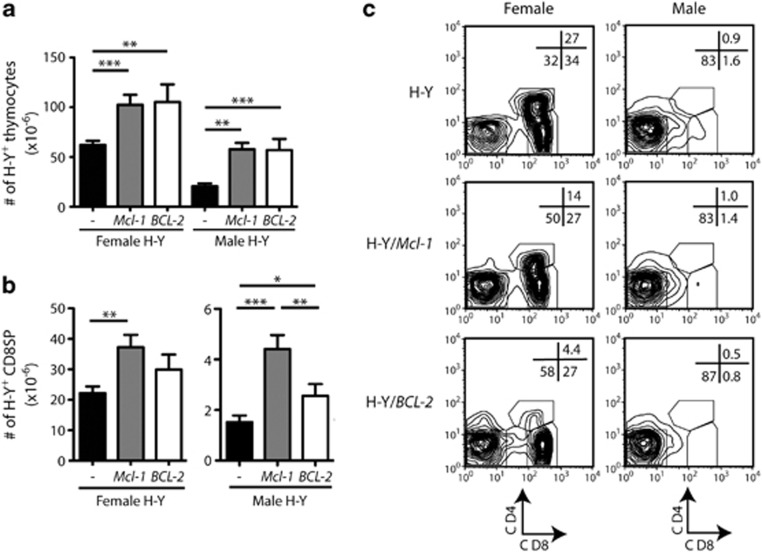
In females, the number of H-Y TCR+ thymocytes was significantly higher in both Mcl-1tg/H-Ytg and BCL-2tg/H-Ytg mice than in control H-Ytg mice (Figure 4a and Table 1), presumably reflecting enhanced positive selection. Interestingly, there were increased numbers of mature H-Y TCR+ CD8SP thymocytes in Mcl-1tg/H-Ytg females, but not in BCL-2tg/H-Ytg females (Figure 4b and Table 1). This difference may reflect the decreased size of the DP population in the latter (Table 1), a still poorly understood consequence of overexpression of Bcl-229 or a specific effect of Mcl-1 overexpression on CD8SP survival. In the spleen, however, the increase in H-Y TCR+ CD8+ T cells was comparable between Mcl-1tg/H-Ytg and BCL-2tg/H-Ytg females (Table 2), suggesting either that comparable numbers of H-Y T cells are ultimately exported from the thymus or that BCL-2 is more effective than Mcl-1 in enhancing the survival of CD8 T cells once they reach the periphery.
Figure 4.
Elevated Mcl-1 alters positive selection and negative selection of thymocytes autoreactive to a ubiquitous self-antigen. (a) Graph of the mean numbers of thymocytes (±S.E.M.) expressing the tg H-Y TCR. (b) Mean numbers of H-Y TCR-positive CD8SP thymocytes (±S.E.M.) (n=6–15/genotype). *P<0.05; **P<0.01; ***P<0.001; Student's t-test. (c). Representative CD4 versus CD8 plots gated on thymocytes expressing the tg H-Y TCR from control (WT), Mcl-1tg (Mcl-1) or BCL-2tg (BCL-2) female and male mice
Table 1. Thymic composition of H-Ytg transgenic mice.
| Group | DN | DP | CD4SP | CD8SP | |
|---|---|---|---|---|---|
| H-Ytg | F | 13±4.7 | 25±9.6 | 2.0±1.8 | 22±8.9 |
| H-Ytg/Mcl-1tg | F | 42±19* | 19±5.4 | 3.5±2.9 | 37±14** |
| H-Ytg/BCL-2tg | F | 61±29* | 8.9±5.1* | 5.0±2.2** | 30±12 |
| H-Ytg | M | 18±7.8 | 0.45±0.25 | 0.86±0.49 | 1.5±0.92 |
| H-Ytg/Mcl-1tg | M | 54±14* | 0.91±0.96 | 2.2±1.9** | 4.4±2.0* |
| H-Ytg/BCL-2tg | M | 50±28* | 0.76±0.61 | 3.3±1.8* | 2.5±1.3*** |
Numbers indicate H-Y TCR+ cells × 106 (mean±SD) in 6–8 week-old mice; n=6–15 mice of each genotype and sex. Significantly different from H-Ytg mice of the same sex *P≤0.001, **P≤0.01, *** P≤0.05.
Table 2. H-Y TCR+ T-cell populations in the spleen of transgenic mice.
| Group | CD4 | CD8 | |
|---|---|---|---|
| H-Ytg | F | 0.86±0.85 | 2.0±2.1 |
| H-Ytg/Mcl-1tg | F | 1.1±0.64 | 5.7±3.3* |
| H-Ytg/BCL-2tg | F | 1.3±0.42 | 5.4±1.7* |
| H-Ytg | M | 0.25±0.21 | 7.5±5.6a |
| H-Ytg/Mcl-1tg | M | 0.65±0.52* | 16±5.0**a |
| H-Ytg/BCL-2tg | M | 0.37±0.21 | 15±10***a |
Numbers indicate H-Y TCR+ cells × 106 (mean±S.D.) in 6–8-week old mice; n=5–17 mice of each genotype and sex. Significantly different from H-Ytg mice of the same sex *P≤0.01, **P≤0.001, ***P≤0.05.
T cells are CD8low
As expected,30 male H-Ytg mice exhibited a dramatic reduction in thymocyte numbers (77% reduction of mean total H-Y TCR+ thymocytes, male versus female) (Figure 4a), mainly due to depletion of DP and CD8SP H-Y+ thymocytes (Figures 4b and c and Table 1). In contrast, Mcl-1tg/H-Ytg and BCL-2tg/H-Ytg males only showed a 44 and 47% reduction in the mean total number of H-Y TCR-positive thymocytes compared with their corresponding female controls (Figures 4a–c and Table 1), suggesting a survival effect additional to that observed in the positive selection conditions (see above). Indeed, the level of protection conferred by expression of the pro-survival transgenes made the total number of H-Y TCR+ thymocytes in male H-Ytg mice comparable to those observed in WT female H-Y TCRtg mice (Figure 4a). Consistent with previous reports for Bcl-2,12, 15 the pro-survival transgenes did not restore the H-Y+ thymocyte subsets to the proportions observed during positive selection (Figure 4c) but instead favoured the ‘escape' of autoreactive thymocytes that had downregulated the CD8 coreceptor (thereby lowering the overall avidity of the TCR/CD3 coreceptor interaction with MHC/self-antigen30). Accordingly, the enhanced rescue of H-Y TCR+ thymocytes from deletion in Mcl-1tg and BCL-2tg mice was most pronounced in the DN subset (Table 1; Figure 4c). Notably, however, the Mcl-1 transgene allowed the survival of more H-Y+ CD8SP than the BCL-2 transgene did (Figure 4b), suggesting that overexpression of Mcl-1 is more effective than BCL-2 at enabling H-Y+ thymocytes to tolerate CD8 expression under conditions of negative selection. Overall, these data suggest that overexpression of either Mcl-1 or BCL-2 had rescued autoreactive H-Y TCR+ thymocytes from deletion.
This rescue was reflected in peripheral lymphoid organs, where increased numbers of H-Y TCR+ CD8+ T cells were observed in Mcl-1tg/H-Ytg as well as BCL-2tg/H-Ytg males (Table 2). The H-Y TCR+ T cells that had escaped thymic deletion in male mice overexpressing Mcl-1 or BCL-2 had reduced levels of the CD8 coreceptor compared with those in WT mice (data not shown), similar to the phenotype of H-Y TCR+ T cells observed in the spleen and lymph nodes of Bim−/− H-Ytg male mice.12
Mcl-1 overexpression alters the selection of thymocytes reactive to tissue-restricted autoantigens
To extend these findings into a system where deletion occurs at the DP-to-SP transition, where most negative selection is thought to occur under physiological conditions, we next tested a model of thymic deletion to a peripheral neo-self-antigen affected by Aire.31 RIP-mOVA tg (hereafter RIP-mOVAtg) mice express membrane-tethered ovalbumin (OVA) under the control of the rat insulin promoter. This transgene drives expression of OVA in the pancreas and kidney but also in epithelial cells of the thymic medulla, where its expression and/or presentation requires Aire.31, 32 When coexpressed with the OT-I TCRαβ transgene (hereafter OT-Itg), which enforces expression of a TCRαβ reactive to OVA257-264 in the context of H2-Kb, the situation is created where thymocytes are deleted in the thymic medulla during the transition from the DP to the CD8SP stage. The OT-I/RIP-mOVA system, therefore, allows investigation of positive and negative selection in an optimal physiological context with regard to both developmental stage and physical location of thymocytes.
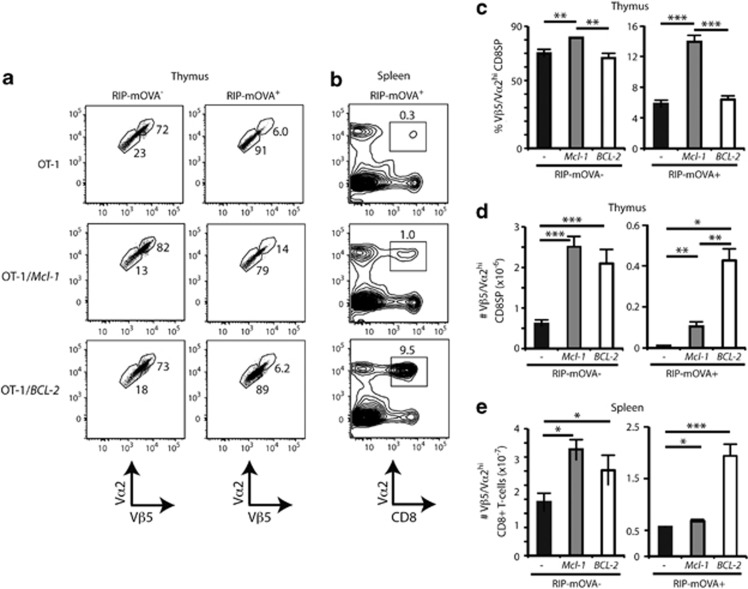
To test the effect of Mcl-1 overexpression in this system, we injected T-cell-depleted bone marrow (BM) from Mcl-1tg/OT-Itg mice into irradiated WT or RIP-mOVAtg mice and assayed thymocyte deletion 8 weeks later. Comparable experiments were also performed with BCL-2tg/OT-Itg mice. By measuring the expression of the Vβ5 and Vα2 components of the OT-I TCR in CD8SP cells, we could determine the maturation state of the OT-I TCR thymocytes under conditions of positive selection (in RIP-mOVA− chimeras) or deletion (in RIP-mOVA+ chimeras) (Figures 5a).
Figure 5.
Mcl-1 overexpression weakly impairs deletion of thymocytes reactive to nonubiquitous self-antigens. (a) Thymocyte plots of TCR levels, gated on Vβ5+ Vα2+ CD8SP from RIP-mOVA− or RIP-mOVA+ chimeras reconstituted with haemopoietic precursors from OT-I TCR tg control (WT), Mcl-1tg or BCL-2tg mice. Regions encompass Vβ5/Vα2high and Vβ5/Vα2low CD8SP thymocytes, with frequencies shown. (b) Splenocyte plots of TCR Vα2 versus CD8 from RIP-mOVA+ chimeras, with percentages of TCR Vα2+ CD8+ T cells shown. Plots representative of three experiments are shown. (c) Graph of the mean proportions (±S.E.M.) of Vβ5/Vα2high CD8SP thymocytes from the chimeras. (d) Mean cell numbers (±S.E.M.) of Vβ5/Vα2high CD8SP thymocytes from the chimeras. (e) Mean cell numbers (±S.E.M.) of Vβ5/Vα2+ CD8+ splenocytes from the chimeras. Data are representative of three experiments and n=4/group. *P<0.05; **P<0.01; ***P<0.001; Student's t-test
In terms of overall cellularity, both the Mcl-1 and BCL-2 transgenes increased the numbers of mature CD8SP OT-I TCR thymocytes under positive selecting conditions (Figure 5d, left panel), consistent with the data from the H-Y TCR system. Furthermore, overexpression of Mcl-1 caused a proportional increase in those CD8SP OT-I TCR thymocytes expressing high levels of Vβ5 and Vα2 (Figures 5a and c), suggesting that mature thymocytes arose or accumulated more frequently within this subset.
In accord with previous reports,31, 32 almost all Vβ5/Vα2hi CD8SP thymocytes were deleted under the negative selecting conditions of OT-I BM/RIP-mOVA+ chimeras (Figures 5a). Expression of the BCL-2 transgene enabled more Vβ5/Vα2hi thymocytes to escape deletion than expression of the Mcl-1 transgene did (Figure 5d, right panel). However, the Mcl-1 transgene engendered a proportional increase in Vβ5/Vα2hi CD8SP in RIP-mOVA+ recipients whereas the BCL-2 transgene did not (Figure 5c, right panel). These data suggest that although Mcl-1 overexpression favours accumulation of the mature Vβ5/Vα2hi CD8SP thymocytes, overexpression of BCL-2 is more protective against deletion than overexpression of Mcl-1, at least to the level afforded by this transgene.
This differential was mirrored in peripheral lymphoid organs. Although a small (but significant) increase in the number of Vβ5/Vα2hi CD8+ T cells was found in the spleens of Mcl-1tg/OT-Itg/RIP-mOVAtg+ chimeras compared with control OT-Itg/RIP-mOVAtg chimeras (Figure 5e), greater numbers were found in the BCL-2tg OT-Itg/RIP-mOVAtg chimeras (Figure 5e).
These data indicate that overexpression of either Mcl-1 or BCL-2 inhibits thymic deletion (Figures 5b and e). Nevertheless, the escaped autoreactive T cells appeared to be held in check by other tolerance mechanisms (e.g., anergy or regulation), as none of the RIP-mOVA+ chimeras expressing either of the two pro-survival transgenes developed diabetes during the 8 weeks they were observed.
We also assayed the effect of Mcl-1 and BCL-2 overexpression on RIP-mOVA-mediated deletion using the OT-II TCR tg system. OT-II tg mice express a TCR that recognises a different OVA peptide (OVA323–339) presented by H2-Ab MHC II molecules, which leads to the differentiation of CD4+ T cells. In an experiment analysing chimeras created by transplantation of OT-IItg, BCL-2tg/OT-IItg or Mcl-tg/OT-IItg BM cells into RIP-mOVAtg or WT mice (n=3-4/group), we observed that elevated BCL-2 or Mcl-1 levels increased positive selection, impaired deletion and increased numbers of peripheral OT-II TCR CD4+ T cells (data not shown), consistent with the results obtained with the OT-I TCR system.
Discussion
The vavP-Mcl-1 transgene provided all major thymocyte subsets with a clear survival advantage during culture (Figures 1a–c), in contrast to results recently reported for a human MCL-1 minigene;33 a differential level and/or pattern of transgene expression may account for this variance. The vavP-Mcl-1 transgene also provided robust protection against diverse cytotoxic insults (etoposide, PMA, ionomycin, dexamethasone), counteracting increases in Bim, Puma, Bmf and Noxa expression (Figures 1c, 2a–f). Protection against dexamethasone-induced death, however, was not as prolonged for thymocytes from the Mcl-1tg mice as for those from vavP-BCL-2tg mice (Figure 1c), apparently due to degradation of Mcl-1 after 24 h (Figure 2). This observation suggests that glucocorticoid treatment may be a useful therapeutic adjuvant for lymphomas and leukaemias overexpressing Mcl-1.
The major goal of this study was to investigate whether overexpression of Mcl-1 perturbed the apoptotic processes that shape the T-cell repertoire. We first tested whether Mcl-1 could protect thymocytes against anti-CD3-mediated deletion, which is driven by elevated expression of Bim.12 Indeed, the protection afforded by overexpression of Mcl-1 was comparable to that provided by overexpression of BCL-2, both in vivo and in vitro (Figure 3), albeit not as great as that found in the absence of Bim.12, 13
We next crossed the Mcl-1tg mice with different TCR tg models developed to investigate positive and negative selection of thymocytes. In the H-Y model,28 expression of the Mcl-1 transgene significantly increased the number of H-Y TCR+ CD8SP thymocytes in female mice whereas expression of the BCL-2 transgene did not (Table 1 and Figure 4c). This finding suggests that overexpression of Mcl-1 has a greater impact upon positive selection than overexpression of BCL-2, and/or that it affects CD8SP survival and accumulation more. The latter scenario is consistent with the high sensitivity of CD8SP to Mcl-1 deletion11 and the inability of Bcl-2 overexpression to fully overcome this requirement.22 Furthermore, mice with reduced thymic Mcl-1 expression had defective positive selection in the H-Y model.34 Taken together, these data suggest that endogenous Mcl-1 levels are limiting for positive selection and important for CD8SP maturation.
Overexpression of Mcl-1 and BCL-2 inhibited deletion in male H-Ytg mice to a similar extent (Figure 4a), primarily by increasing the survival of thymocytes downregulating CD8 (Table 1), a common escape mechanism in this model,28 although CD8SP H-Y TCR+ thymocytes were also elevated.
Similar effects on thymocyte selection were observed in two other TCR transgene settings (OT-I and OT-II), where deletion is induced at the DP-to-SP transition by a neo-antigen (OVA) that requires Aire for its presentation in the medulla.31, 32 Both Mcl-1 and BCL-2 overexpression increased positively selected OT-I thymocytes to a similar extent, although Mcl-1 favoured modest expansion of OT-I CD8SP with high levels of TCR. Whether this proportional increase (consistent with data from the H-Y system) reflects an effect of Mcl-1 overexpression on DP precursors or is intrinsic to the CD8SP compartment might be addressed by conditional overexpression following positive selection. Under negative selecting conditions, overexpression of Mcl-1 was less effective than BCL-2 at rescuing CD8SP thymocytes from deletion in the medulla. Given the results in the H-Y system, these data suggest that the capacity of Mcl-1 overexpression to inhibit apoptosis induced by high-avidity TCR stimulation depends upon the thymocyte subset that is subject to deletion. Such differences would likely reflect the differential expression of proapoptotic Bcl-2 family members by thymocyte subsets. It is also possible that the extent to which Mcl-1 overexpression can inhibit deletion depends on the strength of the TCR stimulus provoked by interaction with cognate peptide–MHC complexes.
Of note, neither BCL-2 nor Mcl-1 overexpression impaired deletion of autoreactive thymocytes to the same extent as Bim deficiency in these same TCR tg models (Bouillet et al.12 and Moran et al.35 our unpublished data). The greater impact of Bim deficiency may reflect its capacity to neutralise all pro-survival Bcl-2 family members, thereby preventing them from blocking activation of proapoptotic Bax and Bak.17, 18 If deletion of autoreactive thymocytes relies on a Bak-dependent step,22 the observation that Bcl-2 binds Bax but not Bak19 could explain why overexpression of BCL-2 does not inhibit deletion as effectively as loss of Bim. As Mcl-1 can interact with both Bak and Bax,19 however, this hypothesis would not readily explain why increased Mcl-1 was not as effective as Bim deficiency, unless the level of expression achieved for this highly unstable protein was insufficient to completely block Bak activation. Indeed, the level of tg Mcl-1 protein in thymocytes of vavP-Mcl-1 mice is at least 2–4 times lower than that of tg BCL-2 protein in vavP-BCL-2 mice. Alternatively, Bim might possess an activity beyond the sequestration of pro-survival Bcl-2 family members that is necessary for Bax and/or Bak activation during thymocyte deletion. Increasing evidence suggests that certain BH3-only proteins, including Bim, can directly activate Bax and Bak via a ‘hit and run' mechanism.36, 37, 38 Pertinently, a recent study of knockin mutant Bim mice suggests that the proapoptotic activity of Bim depends on its ability to engage with both pro-survival proteins and Bax.39
Materials and Methods
Mice
All mice used were on a C57BL/6J background and bred at the Walter and Eliza Hall Institute (WEHI). Experimental protocols were approved by the WEHI Animal Ethics Committee. Tg mouse lines used were vavP-Mcl-1(33)23 and vavP-BCL-2(69),29 which, respectively, express FLAG-tagged mouse Mcl-1 protein and human BCL-2 protein, H-Y TCR,28 OT-I TCR and RIP-mOVA. The vavP tg vector utilises haemopoietic-specific elements of the vav gene.40 The level of tg protein in vavP-BCL-2tg thymocytes is ∼2–4 times higher than that in vavP-Mcl-1tg thymocytes, as judged by western blots performed using BCL-2 and FLAG antibodies and a control lymphoma expressing FLAG-tagged human BCL-2, presumably due to the Mcl-1 protein having a shorter half-life and differences in the copy number or genomic location of the transgene.
Antibodies and flow cytometry
Except where otherwise indicated, antibodies were produced and conjugated to FITC, PE, biotin or APC in our laboratory. Antibodies were: anti-CD4 (YTA3.2.1), anti-CD8 (53.6.7.2), anti-TCRβ (H57.59.1), anti-Vα2 TCR (B20.1; BD Pharmingen, San Diego, CA, USA), anti-Vβ5.1/5.2 TCR (MR9-4; BD Pharmingen) and anti-H-Y TCR (clone T3/70). The thymus or spleen single-cell suspensions were stained with antibody mixtures in 1% v/v fetal calf serum (FCS) in PBS at 4 °C for 20 min, washed, then analysed by flow cytometry using either a FACSCalibur or LSRII (BD Biosciences, San Jose, CA, USA and FloJo software (Tree Star, Ashland, OR, USA)). Intracellular FACS using a biotinylated rat monoclonal FLAG antibody was performed as previously described.23
In vitro survival assays
Thymocytes labelled with fluorochrome-conjugated antibodies were sorted using a MoFlo (Cytomation) high speed sorter, seeded at a density of 20–50 000 cells/well in 96-well flat-bottom plates containing the high-glucose version of Dulbecco modified Eagle medium supplemented with 250 μM asparagine, 50 μM 2-mercaptoethanol and 10% heat-inactivated FCS (Bovogen, Melbourne, VIC, Australia), with or without cytotoxic agents (see figure legends), and cultured at 37 oC. Viability was assessed by flow cytometry after staining with FITC-labelled annexin V plus propidium iodide. For studies of viability following stimulation with antibodies to CD3ɛ (145-2C11) or with antibodies to both CD3ɛ and CD28 (37.N.51), thymocytes were seeded at 2 × 106 cells/well in 6-well plates precoated with antibodies and viability of DP thymocytes was determined at the indicated time points by flow cytometry after staining with propidium iodide, FITC-labelled annexin V, APC-labelled anti-CD4 and PE-labelled anti-CD8.
Treatment with CD3 antibody in vivo
30 μg of CD3 or control antibody (anti-TCRγ; GL3-1A) in 200 μl PBS was injected into the peritoneum of 6–8-week old mice. Thymi were harvested 40 h later and their cellularity and thymocyte subset composition analysed as described above.
Haemopoietic chimeras
BM harvested from OT-Itg, BCL-2tg/OT-Itg or Mcl-1tg/OT-Itg tg mice was depleted of T cells by labelling with biotinylated CD8 and CD3 antibodies, followed by incubation with streptavidin-labelled magnetic microbeads (Miltenyi Biotec, Cologne, Germany), then depletion on an AutoMACS machine using the ‘Deplete_S' programme (Miltenyi Biotec). T-cell-depleted BM cells were then injected i.v. into WT or RIP-mOVAtg mice (1 × 107 cells) that had received a lethal dose of γ-irradiation (2 × 5.5 Gy, 3 h apart). After 1 day, transplanted mice were injected i.p. with 100 μg of Thy-1 antibody (T24/31; WEHI mAb Laboratory, Melbourne, VIC, Australia) daily for 2 days to deplete any residual OT-Itg T cells present in the BM inoculum. Mice were analysed 8 weeks following BM reconstitution.
Immunoblotting
Western blots of thymocyte lysates were probed with antibodies against: Mcl-1 (clone 19C4-15, WEHI mAb Laboratory); β-actin (clone AC-74, Sigma, Sidney, NSW, Australia; loading control); Bim (clone 3C5, ENZO, Exeter, UK); Bcl-2 (clone 3F11, BD Pharmingen); Bid (clone 2D1-3, WEHI mAb Laboratory); Bmf (clone 17A9, WEHI mAb Laboratory); HSP70 (clone N6, W Welch-USCF; loading control); and Puma (AbCam, Cambridge, UK). Detection was performed using HRP-conjugated secondary antibodies and ECL (Amersham Biosciences, Little Chalfont, UK).
PCR analysis
Total RNA (0.2 μg), isolated using RNeasy mini kit (Qiagen, Melbourne, VIC, Australia), was reverse-transcribed using the TaqmanRT system (Roche, Melbourne, VIC, Australia). Real-time PCR analysis was performed on cDNA using QuantiTect SYBR green PCR kit (Qiagen) on an ABI Prism 7900 (Applied Biosystems, Melbourne, VIC, Australia). Relative expression was determined using the comparative threshold cycle method and β-actin as the control. Primer sequences were as follows: β-actin: sense (5′–3′) TATTGGCAACGAGCGGTTC, antisense (5′–3′) CCATACCCAAGAAGGAAGGCT; Noxa: sense (5′–3′) ACTGTGGTTCTGGCGCAGAT, antisense (5′–3′) TTGAGCACACTCGTCCTTCAA.
Acknowledgments
We thank our colleagues C Vandenberg, C Scott, K Heger and P Bouillet for useful discussions; L O'Reilly and D Huang for reagents; J Blyth and F Kupresanin for assistance; B Helbert and C Young for genotyping; K McKenzie, T Camilleri and G Siciliano for mouse husbandry; and WEHI cytometry and histology services. This work was supported by postdoctoral fellowships from EMBO and the Human Frontier in Science Program (KJC); NHMRC Career Development Fellowship (DHDG) (CDF1 #637353); NHMRC Australia Fellowship (AS); research grants from National Health and Medical Research Council (NHMRC) (programme grant 461221) and National Cancer Institute (CA43540); and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Glossary
- TCR
T-cell receptor
- DN
double-negative
- DP
double-positive
- SP
single-positive
- MHC
major histocompatibility complex
- PMA
phorbol 12-myristate 13-acetate
- WT
wild-type
- BM
bone marrow
- tg
transgenic
- OVA
ovalbumin
- RIP
rat insulin promoter
- WEHI
The Walter and Eliza Hall Institute
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by S Nagata
Supplementary Material
References
- Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11:666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11:469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzella F, Tse AGD, Cordell JL, Pulford KAF, Gatter KC, Mason DY. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- Grillot DAM, Merino R, Nuñez G. Bcl-xL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko MM, Punt JA, Bolcavage JM, Levy SL, Allman DM, Cancro MP. Expression of the Bcl-2 family member A1 is developmentally regulated in T cells. Int Immunol. 1999;11:1753–1761. doi: 10.1093/intimm/11.11.1753. [DOI] [PubMed] [Google Scholar]
- Verschelde C, Michonneau D, Trescol-Biemont MC, Berberich I, Schimpl A, Bonnefoy-Berard N. Overexpression of the antiapoptotic protein A1 promotes the survival of double positive thymocytes awaiting positive selection. Cell Death Differ. 2006;13:1213–1221. doi: 10.1038/sj.cdd.4401814. [DOI] [PubMed] [Google Scholar]
- Dzhagalov I, Dunkle A, He YW. The anti-apoptotic bcl-2 family member mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Villunger A, Marsden VS, Zhan Y, Erlacher M, Lew AM, Bouillet P, et al. Negative selection of semimature CD4(+)8(-)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci USA. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci USA. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Dunkle A, Dzhagalov I, He YW. Mcl-1 promotes survival of thymocytes by inhibition of Bak in a pathway separate from Bcl-2. Cell Death Differ. 2010;17:994–1002. doi: 10.1038/cdd.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KJ, Bath ML, Turner ML, Vandenberg CJ, Bouillet P, Metcalf D, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116:3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen TN, McKee A, Wang M, Kushnir E, White J, Refaeli Y, et al. Bim and Bcl-2 mutually affect the expression of the other in T cells. J Immunol. 2007;179:3417–3424. doi: 10.4049/jimmunol.179.6.3417. [DOI] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-xL and Bcl-w are not equivalent targets of ABT-737 and Navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev. Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- De Biasio A, Vrana JA, Zhou P, Qian L, Bieszczad CK, Braley KE, et al. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J Biol Chem. 2007;282:23919–23936. doi: 10.1074/jbc.M700938200. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Ann Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H, Kisielow P. Self-nonself discrimination by T cells. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- Gui J, Morales AJ, Maxey SE, Bessette KA, Ratcliffe NR, Kelly JA, et al. MCL1 increases primitive thymocyte viability in female mice and promotes thymic expansion into adulthood. Int Immunol. 2011;23:647–659. doi: 10.1093/intimm/dxr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Lin NH, Lee JM, Huang CY, Min HJ, Yen JJ, et al. Promoter knock-in mutations reveal a role of Mcl-1 in thymocyte-positive selection and tissue or cell lineage-specific regulation of Mcl-1 expression. J Immunol. 2009;182:2959–2968. doi: 10.4049/jimmunol.0803550. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy S, Metcalf D, Gibson L, Bath ML, Harris AW, Adams JM. Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood. 1999;94:1855–1863. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.