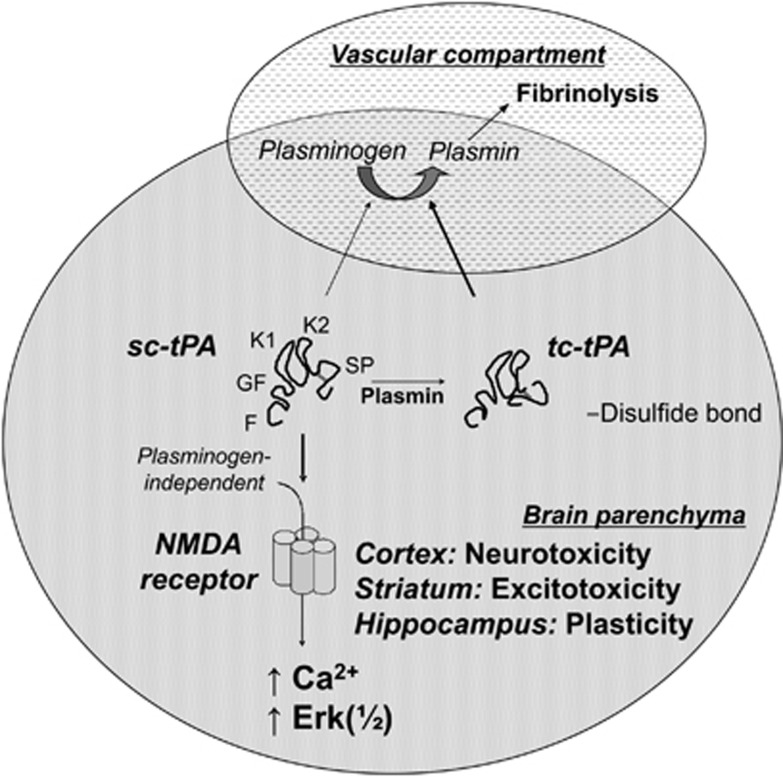
Figure 1.
tPA is a mosaic protein consisting of five distinct modules: a finger domain (F), an epidermal growth factor-like domain (EGF), two kringle domains (K1 and K2) and a serine protease proteolytic domain (SP). Secreted sc-tPA can be converted to its two-chain (tc-tPA) form by plasmin. Both sc-tPA and tc-tPA display an equivalent efficacy for plasmin-induced fibrinolysis in the vascular compartment. In contrast, sc-tPA is the primary effector of plasminogen-independent but proteolytically mediated NMDA-induced neurotoxicity, calcium influx and NMDA-dependent Erk(½) activation on cortical neurons. Similarly, sc-tPA modulates NMDA receptor-induced neuronal plasticity by enhancing long-term potentiation on hippocampal slices