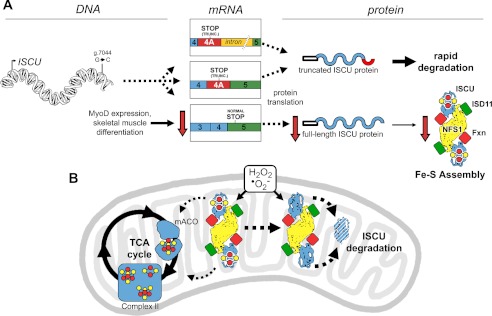
FIGURE 7.
The impact of aberrant ISCU mRNA expression and oxidative stress on ISCU protein synthesis and degradation in ISCU myopathy patient muscle mitochondria. A, an intronic point mutation (g.7044 G→C) leads to decreased expression of the normally spliced ISCU mRNA and the appearance of two predominant patient-specific ISCU mRNA transcripts in all tested patient tissues, both of which encode a truncated (TRUNC.) ISCU protein that is rapidly degraded after synthesis. MyoD expression and maturation of skeletal muscle myofibers lead to further decreases in the expression of normally spliced ISCU mRNA, eventually leading to pathologically low ISCU protein levels in mature skeletal muscles. B, generation of reactive oxygen species such as hydrogen peroxide and superoxide can exacerbate Fe-S cluster deficiency in enzymes and respiratory chain complexes of patient skeletal muscles by causing further depletion of ISCU protein, which cannot be rapidly resynthesized due to the ISCU mRNA splicing defect. TCA cycle, tricarboxylic acid cycle; mACO, mitochondrial aconitase.