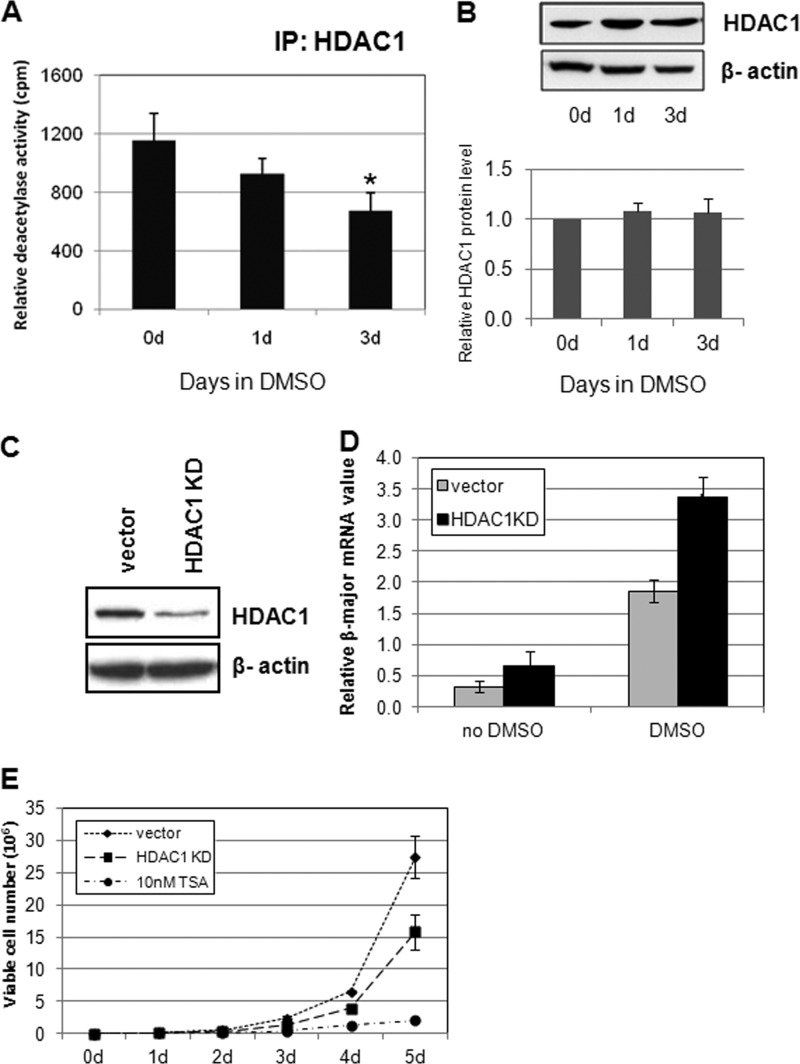
FIGURE 1.
HDAC1 deacetylase activity is down-regulated during erythroid differentiation in MEL cells. A, HDAC1-associated histone deacetylase activity is reduced upon DMSO-induced differentiation of MEL cells. MEL cells were induced with 1.5% DMSO for the indicated days. Nuclear extracts from MEL cells were immunoprecipitated (IP) with HDAC1 antibodies. The deacetylase activities of the immunoprecipitates were determined by deacetylation of 3H-labeled Ac-histone. *, significant difference when compared with day 0 (Student's t test, p < 0.05). B, nuclear extracts from MEL cells were subjected to SDS-PAGE and Western blot analysis with antibodies as indicated. A representative Western blot result is shown. HDAC1 protein level quantification was determined from three independent experiments. C, knockdown of HDAC1 in MEL cells. The siRNA duplexes for mouse HDAC1 were cloned into pSuper retroviral vectors, and the resulting viruses were used to infect MEL cells. Single colonies were selected for the most effective reduction of HDAC1. D, HDAC1 knockdown (KD) affects differentiation. MEL cells with vector, HDAC1, or HDAC2 knockdown constructs were treated with 1.5% DMSO for the indicated time periods. Total RNA was extracted, and real time RT-PCR was performed with β-major globin primers and normalized to β-actin. *, significant difference when compared with vector control (Student's t test, p < 0.01). E, HDAC1 knockdown affects MEL cell proliferation. 1 × 105 control, HDAC1, or HDAC2 knockdown MEL cells were inoculated into medium. The number of viable cells was counted daily for 5 days. The same amount of vector control cells was also treated with 10 nm TSA and tested for proliferation. Data shown are the means ± S.E. of three independent experiments.