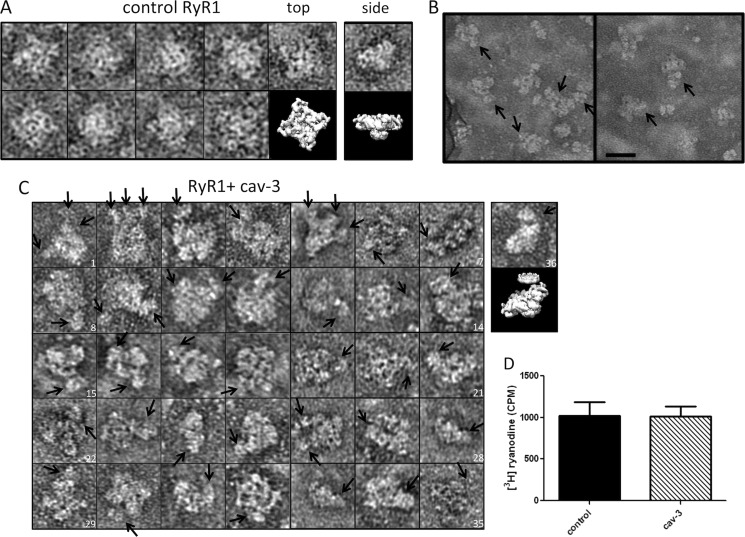
FIGURE 9.
Interaction of solubilized recombinant cav-3 with RyR1. A, control RyR1 complexes presenting the characteristic four-lobed appearance corresponding to a homotetramer with dimensions ∼300 × 300 Å. An EM volume for RyR1 (emd_1275.map) has been modeled to illustrate the RyR1 structure. Although negative staining and adsorption of RyR1 onto the carbon support film is known to lead to a preferred orientation and flattening of the structure and the occasional side view of the protein was observed, an example is shown in the far-right column. B, field of particles with arrows indicating cav-3 bound to RyR1 homotetramers. Scale bar, 50 nm. C, montage of individual RyR1 complexes with cav-3 bound as indicated by the arrows. There is a range of orientations of the RyR1 homotetramers each with at least one cav-3 nonamer attached. In some examples, several cav-3 complexes are associated with a single RyR1. For example, image 2 has three cav-3 nonamers bound, stacked side by side along one edge of a RyR1 tetramer. Image 4 shows one RyR1 complex with a cav-3 nonamer bound to one corner with possibly another cav-3 oligomer attached and linked to several other cav-3 particles. Image 19 shows cav-3, with the circular view of the complex and central cone domain visible, bound to one side of RyR1. The far-right complex (image 36) shows a partial side view of RyR1 with a disc-shaped cav-3 attached to the upper surface. The EM volumes of cav-3 and RyR1 have been modeled in the panel below image 36 to illustrate the association. D, recombinant solubilized full-length cav-3 does not alter [3H]ryanodine binding to skeletal muscle SR membranes. cav-3 was added in molar excess (25:1), with all experiments conducted in triplicate.