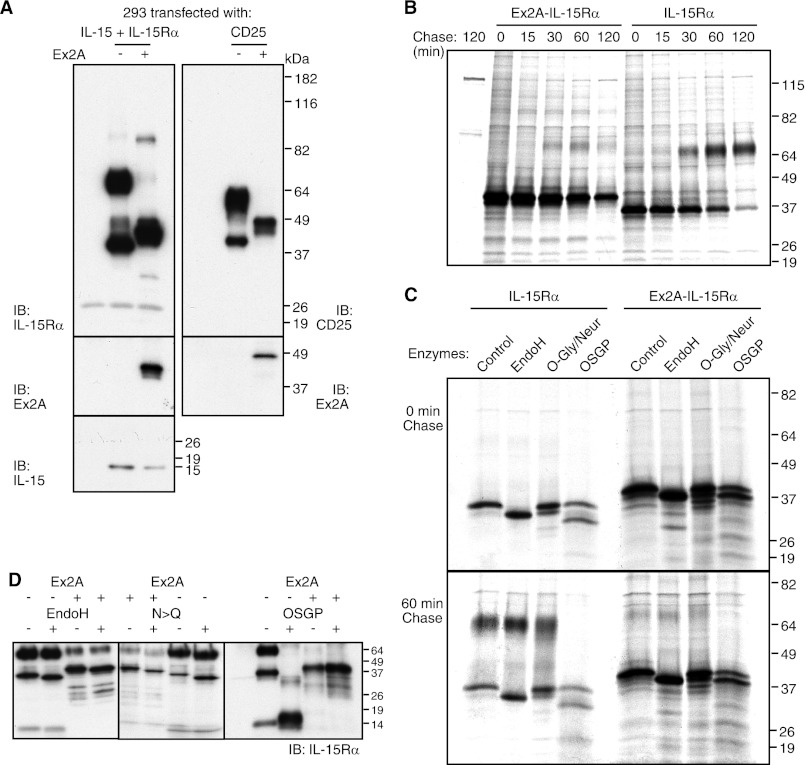
FIGURE 3.
Ex2A affects migratory patterns of IL-15Rα. A, IL-15 and IL-15Rα with or without Ex2A were transfected into 293 cells and analyzed by Western blotting (left panels, the first lane represents a control of untransfected cells). The presence of Ex2A induced the major species of IL-15Rα to migrate at a lower molecular weight (∼42 instead of 65 kDa). Generating a chimeric Ex2A-CD25 protein (upper right panel) similarly caused migratory shifts of CD25. The expression of Ex2A is shown in the two middle panels. IL-15 migrated independently of IL-15Rα at ∼14 kDa (lower left panel). B, pulse-chase experiments revealed that lower molecular weight versions of IL-15Rα are initially generated while an IL-15Rα of higher molecular weight appeared after 30 min of chase only if Ex2A was absent. C, digestions of 35S-labeled proteins showed that a removal of N-linked carbohydrate chains with EndoH caused small shifts of IL-15Rα regardless of Ex2A presence. The high molecular weight version of IL-15Rα that was present after 60 min of chase in the absence of Ex2A was digested by O-sialoglycoprotease (OSGP) but not by O-glycosidase/neuraminidase suggesting an unusual type of modification by O-linked carbohydrate chains. D, similar results were obtained when non-labeled cells were analyzed by Western blotting: The Ex2A domain did not appear to affect N-glycosylation of IL-15Rα since small shifts were induced by EndoH digestions of immunoprecipitated IL-15Rα regardless of Ex2A presence (left), and replacements of the asparagines that are targeted by N-glycosylation also caused IL-15Rα shifts regardless of Ex2A (middle). OSGP cleaved most of IL-15Rα that lacked Ex2A, but the presence of Ex2A prevented this cleavage. The first lane represents an enzyme-only control. Since the activity of this enzyme requires tight clusters of sialylated O-linked oligosaccharides (22), its inability to cleave Ex2A-IL-15Rα suggests that the Ex2A domain prevents O-glycosylation.